Venous access and Ports
Introduction
Totally implantable venous access devices (TIVAD) allow the administration of chemotherapy, antibiotic therapy and parenteral nutrition without limiting the concentration of the fluid or the speed of administration. The port is made of titanium, the septum of silicone. It is a closed system that is totally implanted subcutaneously (figure 1 & 2). They can be used long term (up to 2000 punctures over two years or longer) as long as they are cared for correctly.
The P.A.S. Port is half the length and a third the height of the Port-A-Cath and is often preferred for cosmetic reasons (figure 1). It can be placed on the inner surface of the upper arm where it is more protected from accidental trauma and much less visible. Many adults prefer the smaller, more cosmetically acceptable P.A.S. Port to the larger Port-A-Cath (Burdon et al, 1998).
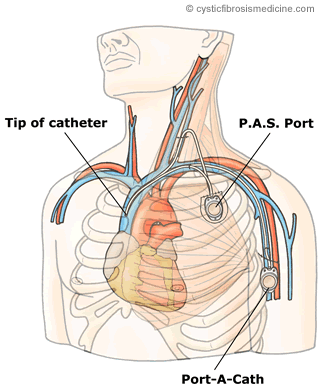
Figure 1. Diagramatic representation of port sites
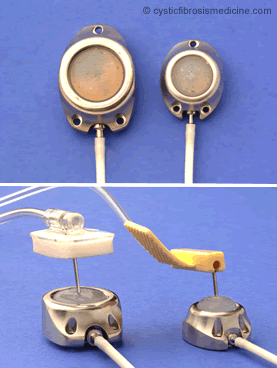
Figure 2. Examples of Port-A-Cath (usually situated under the skin on chest wall) and P.A.S. Port (placed under the skin of the arm). The Ports are accessed using a special needle as shown.
Totally indwelling venous access devices (TIVADs) have proved safe and effective in overcoming problems of venous access in many patients who need regular intravenous antibiotic therapy (Kariyawasam et al, 2000; Leen et al, 2000; Munck et al, 2004; Etherington et al, 2005). It is essential that both family and professionals are familiar with the use of these devices.
Figure 1. Diagramatic representation of port sites
Practicalities of TIVAD use
When it is decided that a patient will benefit from the insertion of a Port-A-Cath or P.A.S. Port, the patient and family will be contacted by the CF Nurse Specialist (CFNS) to discuss the pre- and post–operative care, practicalities of caring for the device and potential complications. This meeting may take place within the hospital or at the patient’s home. The patient and family will have the opportunity to view the various ports and discuss possible insertion sites.
It is essential that all TIVADs are inserted by surgeons with appropriate experience. If a patient receives shared care it is vital that the local hospital is aware of the practicalities of caring for a TIVAD prior to its insertion. Follow up teaching and after care is provided by the CFNS. We recommend that P.A.S. Ports are flushed every four to six weeks and Port-A-Caths every six to eight weeks when not in use, and cared for by staff or carers who are fully trained and have expertise in their everyday care. When in use during intravenous therapy, the needles are replaced weekly.
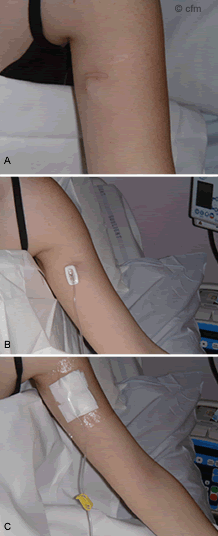
Figure 3. A) P.A.S. Port sited B) P.A.S. Port in upper arm needle in situ C) P.A.S. Port protective dressing and needle in situ.
General Principles
Always use aseptic technique when handling the port, giving medication or taking blood samples.
Only access the port when strictly necessary for the patient’s treatment – the less the intravenous line is handled, the less infection risk there is.
Only use the correct access needles (huber point).
Always maintain a closed system to prevent infection or an air embolism.
Never access the port unless you have been shown how.
Never mix bolus drugs or infusions in the line, always flush with saline in between (SASH: saline, administer medication, saline, heparin).
Always use a 10ml or larger syringe – smaller syringes exert high pressures and risk damaging the line. Do not exceed a pressure of 40 psi.
Accessing the device
Always access the port at an angle of 90 degrees to the septum (figure 2 & 3).
Insert the needle steadily until you feel the bottom of the port.
Avoid excessive pressure on the needle once it has reached the bottom of the port.
Needle length – should be long enough to reach the bottom of the port when inserted. When using the GRIPPER needle or the winged infusion set, the needle hub should be flush against the skin surface.
To avoid injection into the sub-cutaneous tissue, once the septum is punctured, do not tilt or rock the needle as this may cause fluid leakage or damage to the septum.
Blood sampling
Withdraw at least 5ml of blood and discard.
Withdraw the desired amount of blood.
Immediately flush the system with 20ml of 0.9% saline.
Instill 5ml of heparin (100iu/ml) establishing a heparin lock. Maintain positive pressure by clamping the extension set whilst injecting the last 0.5ml.
Precautions
Sports
If the patient is involved with activities or occupations which involve excessive or repetitive upper extremity and/or shoulder or pectoral girdle motion they should be informed that they may increase the possibility of catheter fragmentation due to compression of the catheter between the clavicle and the first rib (The pinch-off syndrome).
Medical procedures
These should be restricted on the patient’s arm in which the system is implanted. Don’t withdraw blood from/or infuse medication into the arm in which the implanted system is located in unless you are using the port. Inadvertent puncture of the catheter will result in catheter damage. Avoid measuring the patient’s blood pressure on this arm, since catheter occlusion or damage to the catheter could occur.
Dressing and needle change
Centres for disease control and prevention guidelines recommend changing the dressing whenever it is soiled, damp or loose.
Needles should be changed every seven days. However, there is some evidence that it is safe to leave needles in for two weeks without changing it although In the Leeds Units this resulted in a significantly higher incidence of port infections (unpublished data).
Trouble shooting
Difficulty in flushing/infusing: Possible causes and possible solutions.
Clamps may be closed. Open clamp.
Catheter may be wedged in a smaller vessel or tip may be pressed against the vessel wall. Have the patient lie flat and get them to move their shoulders and rotate their head. Check flow. If resistance is still detected, blockage may be caused by drug precipitation, fibrin deposition at the catheter tip, intraluminal clot or a pinched catheter. If it is a chest placed system, the catheter may be kinked or ‘pinch- off’ syndrome may be occurring. Sign of this – unable to flush or aspirate fluid when the patient is sitting, but aspirates and flushes easily when lying down.
Needle may not be inserted completely or may be too short to pass through the septum to the bottom of the port. Insert the needle steadily through the septum or re-access the port with a longer needle. Insert the needle completely through the portal septum at a 90 degree angle to the port.
Catheter may be occluded because of drug precipitate, fibrin deposition at the catheter tip or an intraluminal clot. Using a 10ml syringe, flush with 0.9% saline alternating between irrigation and aspiration. If resistance is still felt and thrombosis is suspected consider Urokinase. See Urokinase in drug database.
Difficulty aspirating blood: as above.
Pain
Portal pocket or catheter insertion site. Check for drainage. Discharge at the insertion site. Port may be infected. Assess the patient for other signs or symptoms of infection.
Vein may be irritated because of implantation technique, infusion of acidic or alkaline solutions, or infected vein related to contaminated catheters.
Needle may have been accidentally pulled. Re-evaluate the site. Fluid may be infusing into the tissue.
Catheter may have ruptured. Diagnosed by contrast fluid into the tissues. Undertake a portagram.
Moisture at or around the insertion site
Dressing may have been exposed to water or excessive perspiration. Inquire about recent activity which may explain dampness of the dressing.
Extension set may be loose. Check connection between the needle extensions and tighten connectors.
Needle may be displaced. Check position of the needle ensure correct needle length. Check that dressing adequate to secure the needle.
Needle may have been accidentally inserted into the surrounding tissue.
Septum integrity may have been compromised. Notify physician of leaking from the port. May need contrast study to confirm this.
Complications
Potential Complications
Air embolism, artery or vein puncture, arteriovenous fistula Brachial plexus injury, cardiac arrhythmia, cardiac puncture, cardiac tamponade, catheter disconnection or fragmentation with possible embolism of catheter, catheter occlusion, catheter rupture, drug extravasion, erosion of portal/catheter through the skin and/or a blood vessel, fibrin sheath formation at the catheter tip, haematoma, haemathorax, implant rejection, infection/bacteremia/sepsis, migration of port/catheter, pneumothorax, thoracic duct injury, thromboembolism, thrombosis, thrombophebitis. These complications also apply to the Port-A-Cath system.
When cared for properly, complications are rare, although patients with diabetes mellitus need to pay particular attention to aseptic technique and blood sugar control (Horn & Conway, 1993). Line infections are a recognised complication (Kariyawasam et al, 2000; Ratnalingham et al, 2002).
We have reported an eight year experience of TIVAD use in our adult patients (Etherington et al, 2005). One hundred and sixty eight TIVADs were placed in 128 patients. The total number of catheter days was 161,832. The average catheter lifespan was 963 days. There were 57 catheter related complications, an overall incidence of 33.5% or one complication per 2,840 catheter days. This figure is lower than in other reports (Munck et al, 2004).
The complication rates for individual problems were as follows: infection (11%), catheter blockage (7%), leakage (5%), vascular thrombosis (4%), detached and migrating catheter (4%), malposition (2%), pneumothorax (1%) and fractured line (<1%).
Sixty-one (36%) devices were removed for TIVAD related complications.
Key points
• TIVADs provide safe, effective and convenient long term intravenous access
• There may be important complications
• TIVADs should be inserted by surgeons with appropriate experience
• If a patient receives shared care the local hospital must know how to care for a TIVAD
• Follow up teaching and after care should be provided by the CF Centre CFNS
• P.A.S. Ports should be flushed every four to six weeks and Port-A-Caths every six to eight weeks when not in use
References
Burdon J, Conway SP, Murchan P, et al. Five years experience of the PAS Port intravenous access system in adult cystic fibrosis. Eur Respir J 1998; 12: 212-216. [PubMed]
Etherington C, Jones E, Peckham D, et al. A review of 168 totally implantable venous access devices [TIVAD’s] inserted over an 8 year period [1995-2002] in a Regional Adult CF Centre. J Cyst Fibros 2005; 4(Suppl 1): S14.
Horn CK, Conway SP. Candidaemia: risk factors in patients with cystic fibrosis who have totally implantable venous access systems. J Infect 1993; 26: 127-132. [PubMed]
Kariyawasam HH, Pepper JR, Hodson ME, et al. Experience of totally implantable access devices (TIVADs) in adults with cystic fibrosis over a 13 year period. Respir Med 2000; 94: 1161-1165. [PubMed]
Leen G, Greally P, Dass GL, et al. Totally implantable vascular access devices (TIVADs) in cystic fibrosis: a 10 year experience in 41 patients. Abstract Book, XIII International CF Congress, Stockholm 2000: 167.
Munck A, Malbezin S, Bloch J, et al. A follow-up of 452 totally implantable vascular devices in cystic fibrosis patients. Eur Respir J 2004; 23: 430-434. [PubMed]
Ratnalingham RA, Peckham D, Denton M, et al. Stenotrophomonas maltophilia bacteraemia in two patients with cystic fibrosis associated with totally implantable venous access devices. J Infect 2002; 44: 53-55. [PubMed]
