FATTY ACIDS (please see also NUTRITION AND GROWTH TOPIC)
1962 Kuo PT, Huang NN, Bassett DR. The fatty acid composition of the serum chylomicrons and adipose tissue of children with cystic fibrosis of the pancreas. J Pediatr 1962; 60:394-403. [PubMed]
The first study of fatty acids in blood and tissue lipids of patients with CF. The fatty acid composition of chylomicrons and adipose tissue from children with CF who had variable degrees of fat malabsorption was compared with the values from controls. There was a relative decrease in linoleic acid and increased palmitoleic and oleic acids. Subsequently the abnormalities have been explained as related to liver disease, the basic defect and the intestinal malabsorption. Most recently an association with the presence of diseased infected lungs had been described as the values normalise after lung transplantation (Witters et al, 2013 below).

Bob Elliot
Prof. Bob Elliott and colleagues from New Zealand published several papers showing improvement in the clinical state with supplements of medium chain triglycerides even to the extent of returning the sweat electrolytes to nearer normal values (Elliott RB. Aust Paediatr J 1972 below; 8:217; Elliott RB, Robinson PG. Arch Dis Child 1975; 50:75-78; Elliot RB. 1976; 57:474-479). However, subsequent studies failed to substantiate their findings (Davidson GP et al. Aust Paediatr J 1978; 14:80-82; Chase et al. Pediatrics 1979; 59:428-432 below)
1972 Elliott RB. The effect of essential fatty acid on sweat sodium concentrations in cystic fibrosis. Aust Paediatr J 1972; 8:217
Prof. Bob Elliott and colleagues from Auckland, New Zealand published several papers showing improvement in the clinical state when children with CF were supplemented with medium chain triglycerides even to the extent of returning the sweat electrolytes to nearer normal values (also Elliott RB, Robinson PG. Arch Dis Child 1975; 50:75-78 below; Elliott RB. Pediatrics 1976; 57:474-479 below).
There was considerable discussion as to whether a disturbance of fatty acids resulted in an abnormality of prostaglandins. Rivers JA & Hassam AG suggested an abnormality of fatty acid metabolism such that there was a need for increased linoleic acid (Lancet 1975; ii: 642-643). Subsequent studies failed to substantiate their findings (Davidson GP et al. Aust Paediatr J 1978; 14:80-82; Chase et al. Pediatrics 1979; 59:428-432).
1975 Elliott RB, Robinson PG. Unusual course in a child with cystic fibrosis treated with fat emulsion. Arch Dis Child 1975; 50:76-78. [PubMed]
A child with CF received regular intravenous infusions of soya oil emulsion from the first weeks. The authors state that “Sweat tests improved, pancreatic achylia was relieved and the child at present remains entirely well. Correction of fatty acid found in cystic fibrosis may prevent some of the manifestations of the disease”.
Elliott and colleagues from Auckland, New Zealand published several papers on this subject the first showing improvement in the clinical state with supplements of medium chain triglycerides even to the extent of returning the sweat electrolytes to nearer normal values (Elliott RB. Aust Paediatr J 1972; 8:217; Elliot RB. 1976; 57:474-479). Unfortunately, subsequent studies failed to substantiate their earlier findings (Davidson GP et al. Aust Paediatr J 1978; 14:80-82; Chase et al. Pediatrics 1979; 59:428-432 below)
1978 Chase HP, Dupont J. Abnormal levels of prostaglandins and fatty acids in the blood of children with Cystic Fibrosis. Lancet 1978; ii: 236-238. [PubMed]
Low levels of linoleic acid in 12 children with CF and higher production of prostaglandin F2 than controls, were corrected by linoleic acid supplements (see Chase et al, 1979 below)..
1979 Chase HP, Cotton EK, Elliot RB. Intravenous linoleic acid supplementation in children with cystic fibrosis. Pediatrics 1979; 64:207-213. [PubMed]
This study was performed to investigate the previous observation of Prof. Bob Elliott of clinical improvement after IV Intralipid (Elliott & Robinson, 1975, above). On alternate weeks for a year 10 children received either intravenous Intralipid or a similar number of calories as 10% glucose. The Intralipid-treated group were marginally better re. height but the numbers were small and the differences were not significant. The authors thought the differences may have been obscured by the response of all patients to the increased attention they received during the trial. The authors suggested multicentre studies from an early age and also that all should be screened annually for low linoleic acid levels and supplementing those with levels below 2SD of normal.
This was perhaps the first double blind study of a nutritional intervention in cystic fibrosis. Unfortunately the present findings did not confirm Elliot & Robinson’s 1975 observation and did not seem to have a significant impact on CF management at the time. The questions surrounding the importance of fatty acid deficiency in CF would persist for many decades and the subject is still an area of uncertainty and investigation.
1999 Freedman SD Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr-/-mice. PNAS 1999; 96:13995-14000.[PubMed]
A deficiency in essential fatty acid metabolism has been reported previously in plasma from patients with cystic fibrosis. The objective of this present study was to determine whether alterations in fatty acid metabolism were specific to CF-regulated organs and whether they played a role in the expression of disease. A membrane lipid imbalance was found in ileum, pancreas, and lung from cftr(-/-) mice characterized by an increase in phospholipid-bound arachidonic acid and a decrease in phospholipid-bound docosahexaenoic acid (DHA). This lipid imbalance was observed in organs pathologically affected by CF including lung, pancreas, and ileum and was not secondary to impaired intestinal absorption or hepatic biosynthesis of DHA. As proof of concept, oral administration of DHA to cftr (-/-) mice corrected this lipid imbalance and reversed the observed pathological manifestations in the pancreas. The authors considered these results strongly suggest that certain phenotypic manifestations of CF may result from remediable alterations in phospholipid-bound arachidonic acid and DHA levels (figure 1).
– This paper, caused considerable interest in medical circles and in the media at the 1999 North American CF Conference in Seattle, as Dr Freedman of the Beth Israel Deaconess Medical Centre Boston, suggested that essential fatty acid imbalance, affected the phenotypic expression of the CF defect and hence implied that the CF phenotype could be modified by correction of the imbalance. This latest revival in interest in essential fatty acids was made possible by the availability of CF mice and the opportunity to examine their pancreatic tissue.
Unfortunately subsequent studies by this group failed to confirm the fundamental importance of these findings (Beharry et al, 2007 below) but did suggest that DHA therapy may release endogenous inhibitors of inflammation (below) although it is fair to say that the initial enthusiasm has waned (Freedman SD et al, 2004; Beharry et al, 2007 both described out of chronological order for convenience below).
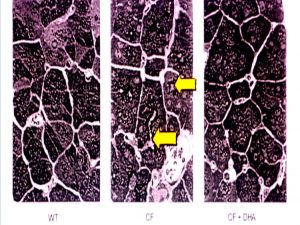 |
| Pancreatic sections. Left – wild type mice. Centre – cftr-/- mice. Right – cftr -/- mice on DHA. (Shown at the 1999 North American CF Conference). |
2004 Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O’Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Eng J Med 2004; 350:560-569. [PubMed]
The authors previously demonstrated that arachidonic acid levels are increased and docosahexaenoic acid levels are decreased in affected tissues from cystic fibrosis-knockout mice (Freedman et al, 1999 above). In this present study of fatty acids from nasal- and rectal-biopsy specimens, nasal epithelial scrapings, and plasma were analyzed from 38 subjects with CF and compared with results in 13 obligate heterozygotes, 24 healthy controls, 11 subjects with inflammatory bowel disease, 9 subjects with upper respiratory tract infection, and 16 subjects with asthma. The ratio of arachidonic to docosahexaenoic acid was increased in mucosal and submucosal nasal-biopsy specimens (P<0.001) and rectal-biopsy specimens (P=0.009) from subjects with CF compared with the healthy control subjects. In nasal tissue, this change reflected an increase in arachidonic acid levels and a decrease in docosahexaenoic acid levels. In cells from nasal mucosa, the ratio of arachidonic to docosahexaenoic acid was increased in subjects with cystic fibrosis (P<0.001), as compared with healthy controls, with values in obligate heterozygotes intermediate between these two groups (P<0.001). The authors concluded that these data indicated that alterations in fatty acids similar to those in cystic fibrosis-knockout mice are present in CFTR-expressing tissue from subjects with cystic fibrosis.
Despite considerable published work, up to 2008, EFA therapy has not been established as beneficial in people with CF; nor has this work had a major impact on the understanding of or treatment of cystic fibrosis as was hoped when first reported in 1999.
Lloyd-Still JD, Powers CA, Hoffman DR, Boyd-Trull K, Lester LA, Benisek DC, Arterburn LM. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition 2006; 22:36-46. [PubMed] Several studies have reported omega-3 and omega-6 fatty acid imbalances in patients with cystic fibrosis. Whether these imbalances contribute to, or are manifestations of, the pathophysiology of CF is unknown. This study by John Lloyd-Still and colleagues from Chicago was to determine bioavailability, tissue accretion, and safety of a large dose of an algal source of docosahexaenoic acid (DHA) triacylglycerol and to observe the effects on lung function in patients with CF. Twenty subjects with CF (8 to 20 yrs of age) were randomly assigned to receive algal oil providing 50 mg of DHA per kilogram per day (1 to 4.2 g of DHA per subject per day) or placebo for 6 months. The authors found that algal DHA triacylglycerol oil is readily absorbed well tolerated, and increases blood and tissue DHA levels in patients with CF. No adverse developments were associated with this large dose of DHA oil.
The authors concluded that larger studies of longer duration are needed to determine whether DHA supplementation results in any clinically significant benefits in patients with CF. Subsequently a report from Belgium failed to show any clinical improvement after a year’s supplementation with a DHA rich algal oil (Van Biervliet S et al. Prostaglandins Leukot Essent Fatty Acids 2008; 78:109-115. [PubMed]).
Dr John Lloyd Still is one of the leading figures in CF care and research in N. America and has been involved in CF and paediatric gastroenterology for many years since he qualified at Guys Hospital in London in 1960. After qualifying he worked in London in paediatrics and eventually moved to Boston where he worked with Harry Shwachman. He edited a major textbook on CF in 1983 to which most of the leading authorities on CF in North America at the time contributed (Textbook of Cystic Fibrosis. Lloyd-Still J D. John Wright PSG Inc. 1983). He is particularly interested in, and has published widely on, the gastroenterological and nutritional aspects of CF.
2007 Beharry S, Ackerley C, Corey M, Kent G, Heng YM, Christensen H, Luk C, Yantiss RK, Nasser IA, Zaman M, Freedman SD, Durie PR. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol – Gastr L 2007; 292:G839-48. [PubMed]
A congenic C57Bl/6J cystic fibrosis transmembrane conductance regulator (Cftr)(-/-) mouse model, which develops cystic fibrosis (CF)-like pathology in all organs, was used to evaluate the short- and long-term therapeutic effects of dietary docosahexaenoic acid (DHA). Thirty-day-old Cftr (-/-) mice and wild-type littermates were randomized to receive a liquid diet with or without DHA (40 mg/day). Animals were killed for histological and lipid analysis after 7, 30, and 60 days of therapy. DHA had no significant therapeutic or harmful effect on the lung, pancreas, or ileum of the Cftr (-/-) mice or their wild-type littermates. In contrast, dietary DHA resulted in highly significant amelioration of the severity of liver disease in the Cftr (-/-) mice, primarily a reduction in the degree of peri-portal inflammation. The authors concluded that inhibition of cytokines and/or eicosanoid metabolism and release of endogenous inhibitors of inflammation by the DHA may account for the anti-inflammatory effects in the liver of this congenic murine model of CF. The potential therapeutic benefits of DHA in severe CF-associated liver disease remain to be explored.
– So the EFA story continues and although dietary DHA supplements had no effect on many organs of the Cftr (-/-) mouse including the pancreas as had been suggested in 1999, there was considerably less hepatic inflammation in the treated mice.
2000 Bhura-Bandali FN, Suh M, Man SF, Clandinin MT. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator alters control of essential fatty acid utilization in epithelial cells. J Nutr 2000; 130:2870-2875. [PubMed]
Essential fatty acid (EFA) incorporation into phospholipid is influenced by chloride channels, suggesting that CFTR may exert a regulatory effect on EFA metabolism. The authors state that the observations in this paper suggest that CF results in a defect in the utilization of 18:2(n-6), which they attribute in part to the defective CFTR.
– This is yet another twist to the EFA story in cystic fibrosis. Recently Freedman et al (1999 above) had shown a membrane lipid imbalance in the ileum, pancreas, and lung from cftr (-/-) mice characterized by an increase in phospholipid-bound arachidonic acid and a decrease in phospholipid-bound docosahexaenoic acid. This finding caused considerable interest at the 1999 North American CF Conference but unfortunately subsequent studies failed to confirm the fundamental importance of the findings. A later report from Freedman and colleagues in 2007 suggested that DHA therapy may release endogenous inhibitors of inflammation although the initial enthusiasm has waned (Freedman SD et al, 2004; Beharry et al, 2007 both below).
2001 Strandvik B, Gronowitz E, Enlund F, Martinsson T, Wahlstrom J. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J Pediatr 2001; 139:650-655.[PubMed]
Serum concentrations of linoleic acid and docosahexaenoic acid were significantly lower in patients with severe cystic fibrosis transmembrane conductance regulator mutations, such as DF508, suggesting an association between the basic defect and abnormal essential fatty acid metabolism in CF patients. A relationship between these fatty acids and the basic defect had been suggested previously – most recently by Freeman et al, (1999 above).

Birgitta Strandvik
Birgitta Strandvik, MD, PhD, (figure ) is Professor Emeritus of Pediatrics at Goteborg University, Sweden. She has published more than 250 scientific papers and book chapters and made major contributions to the scientific and clinical aspects of both cystic fibrosis and paediatric gastroenterology. She has a particular interest in essential fatty acids but has always been obviously greatly concerned in the practical care of patients with CF.
2004 Freedman SD. Blanco PG. Zaman MM. Shea JC. Ollero M. Hopper IK. Weed DA. Gelrud A. Regan MM. Laposata M. Alvarez JG. O’Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Eng J Med 2004; 350:560-569. [PubMed]
The ratio of arachidonic to docosahexaenoic acid was increased in mucosal and submucosal nasal-biopsy specimens (P<0.001) and rectal-biopsy specimens (P=0.009) from subjects with cystic fibrosis and pancreatic sufficiency and subjects with cystic fibrosis and pancreatic insufficiency, as compared with values in healthy control subjects. So alterations in fatty acids similar to those in cystic fibrosis-knockout mice are present in CFTR-expressing tissue from subjects with cystic fibrosis.
2006 Lloyd-Still JD, Powers CA, Hoffman DR, Boyd-Trull K, Lester LA, Benisek DC, Arterburn LM. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition 2006; 22:36-46.[PubMed]
Several studies have reported omega-3 and omega-6 fatty acid imbalances in patients with cystic fibrosis. Whether these imbalances contribute to, or are manifestations of, the pathophysiology of CF is unknown. This study by John Lloyd-Still and colleagues from Chicago was to determine bioavailability, tissue accretion, and safety of a large dose of an algal source of docosahexaenoic acid (DHA) triacylglycerol and to observe the effects on lung function in patients with CF.
Twenty subjects with CF (8 to 20 yrs of age) were randomly assigned to receive algal oil providing 50 mg of DHA per kilogram per day (1 to 4.2 g of DHA per subject per day) or placebo for 6 months. The authors found that algal DHA triacylglycerol oil is readily absorbed well tolerated, and increases blood and tissue DHA levels in patients with CF. No adverse developments were associated with this large dose of DHA oil.
The authors concluded that larger studies of longer duration are needed to determine whether DHA supplementation results in any clinically significant benefits in patients with CF. Subsequently a report from Belgium failed to show any clinical improvement after a year’s supplementation with a DHA rich algal oil (Van Biervliet S et al. Prostaglandins Leukot Essent Fatty Acids 2008; 78:109-115. [PubMed]).
 |
|
| John Lloyd-Still |
Dr John Lloyd Still (figure) is one of the leading figures in CF care and research in N. America and has been involved in CF and paediatric gastroenterology for many years since he qualified at Guys Hospital in London in 1960. After qualifying he worked in London in paediatrics and eventually moved to Boston where he worked with Harry Shwachman. He edited a major textbook on CF in 1983 to which most of the leading authorities on CF in North America at the time contributed (Textbook of Cystic Fibrosis. Lloyd-Still J D. John Wright PSG Inc. 1983). He is particularly interested in, and has published widely on, the gastroenterological and nutritional aspects of CF
2008 Van Biervliet S, Devos M, Delhaye T, Van Biervliet JP, Robberecht E, Christophe A. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukotr Essent Fatty Acids 2008; 78:109-115. [PubMed]
The treatment group was supplemented with algal DHA-rich oil and the control group with sunflower seed oil. There was no difference between the control and treatment groups for percentage weight for height, caloric intake, FEV1% and FVC% at the start of the study and after 1 year of supplements.
– So although DHA-rich oil shifted the serum phospholipid fatty acids to a less pro-inflammatory profile, no conclusive clinical improvement could be observed.
2010 Strandvik B. Fatty acid metabolism in cystic fibrosis. [Review] Prostag Leukotr Ess 2010; 83:121-129. [PubMed]
Twenty years ago the gene responsible for cystic fibrosis transmembrane conductance regulator (CFTR), the protein defective in cystic fibrosis (CF), was identified but research of this monogenetic disease has not provided an explanation for the divergent symptoms, and a treatment breakthrough is still awaited. This review discusses different aspects of disturbances in lipid metabolism seen in CF.
These include increased release of arachidonic acid (AA) from cell membrane phospholipids and a low status of linoleic and docosahexaenoic acids. Recent research has explored more complicated lipid associations. Disturbances in annexins and ceramides might act in concert to explain the impact on inflammation and AA release. The connections to CFTR and between the disturbances in essential fatty acid metabolism are reviewed. The metabolic interactions, some of which might be compensating, possibly explain the difficulties in understanding the fatty acid disturbances in relation to different symptoms and their relation to the defective CFTR.
Brigitta Strandvik (figure) has had a particular interest in fatty acid metabolism in CF for many years and in this article by an expert in the subject, brings together some of the existing knowledge; also there does seem to be some interesting and possibly significant associations such as that with ceramides.
2010 Zaman MM, Martin CR, Andersson C, Bhutta AQ, Cluette-Brown JE, Laposata M, Freedman SD. Linoleic acid supplementation results in increased arachidonic acid and eicosanoid production in CF airway cells and in cftr-/- transgenic mice. Am J Physiol – Lung C 2010; 299:L599-606. [PubMed]
Cystic fibrosis (CF) patients display a fatty acid imbalance characterized by low linoleic acid levels and variable changes in arachidonic acid. This led to the recommendation that CF patients consume a high-fat diet containing >6% linoleic acid. The authors hypothesized that increased conversion of linoleic acid to arachidonic acid in CF leads to increased levels of arachidonate-derived pro inflammatory metabolites and that this process is exacerbated by increasing linoleic acid levels in the diet. To test this hypothesis, they determined the effect of linoleic acid supplementation on downstream pro inflammatory biomarkers in two CF models: 1) in vitro cell culture model using 16HBE14o(-) sense [wild-type (WT)] and antisense (CF) human airway epithelial cells; and 2) in an in vivo model using cftr(-/-) transgenic mice. Fatty acids were analyzed by gas chromatography-mass spectrometry (GC/MS), and IL-8 and eicosanoids were measured by ELISA. Neutrophils were quantified in bronchoalveolar lavage fluid from knockout mice following linoleic acid supplementation and exposure to aerosolized Pseudomonas LPS. Linoleic acid supplementation increased arachidonic acid levels in CF but not WT cells. IL-8, PGE(2), and PGF(2alpha) secretion were increased in CF compared with WT cells, with a further increase following linoleic acid supplementation. cftr(-/-) Mice supplemented with 100 mg of linoleic acid had increased arachidonic acid levels in lung tissue associated with increased neutrophil infiltration into the airway compared with control mice. These findings support the hypothesis that increasing linoleic acid levels in the setting of loss of cystic fibrosis transmembrane conductance regulator (CFTR) function leads to increased arachidonic acid levels and pro inflammatory mediators.
– These findings are important as there was considerable interest in linoleic acid supplementation in people with CF – even a trial of intravenous linoleic acid in the Seventies. The situation regarding essential fatty acids remain unclear to this writer!
2010 Rhodes B, Nash EF, Tullis E, Pencharz PB, Brotherwood M, Dupuis A, Stephenson A. Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros 2010; 9:24-28. [PubMed]
A high fat high calorie diet is advocated for patients with cystic fibrosis (CF) however the lipid profiles of individuals with CF, including those with CF-related diabetes (CFRD), are not well studied. We conducted a retrospective review of adult CF patients attending St Michael’s Hospital between January 2005 and December 2007. 334 patients (77% pancreatic insufficient (PI)) were included in the study. Mean HDL cholesterol was significantly lower in males (p<0. 0001) with 44% of males having HDL cholesterol <38. 7mg/dL(1mmol/L). Pancreatic sufficient patients were more likely than PI subjects to have total cholesterol >201mg/dL(5. 2mmol/L) (p<0. 01). 5% of subjects had triglyceride concentrations >195mg/dL(2. 2mmol/L). Diabetes was diagnosed in 23% of subjects. Lipid profiles were similar between diabetics and non-diabetics. Total cholesterol and triglycerides both increased with increasing age and increasing BMI (p<0. 01).
The authors concluded that dyslipidemia occurs in CF patients however no differences in lipid profiles were seen between those with diabetes and those without. Fasting lipids should be monitored in CF patients, particularly those with PS, older age, and high BMI. As survival in CF increases, the prevalence of dyslipidemia may increase resulting in clinically important complications.
2011 Becker KA. Henry B. Ziobro R. Riethmuller J. Gulbins E. Lipids in cystic fibrosis. [Review] Exp Rev Respir Med 2011; 5:527-535.[PubMed]
Recent studies developed novel and exciting concepts regarding the pathogenesis and treatment of cystic fibrosis. In particular, several studies indicated a critical role of death receptors, caveolae proteins, membrane rafts, alterations of the ceramide metabolism with an accumulation of ceramide and a reduction of 15-keto-prostaglandin 2 in cystic fibrosis lungs. These alterations have been found to be critically involved in the pulmonary inflammation and infection susceptibility of cystic fibrosis patients. However, albeit these studies provided novel insights into molecular mechanisms causing inflammation and vulnerability to infection, the details of these processes are still unknown
2011 Oliver C, Jahnke N. Omego-3 fatty scids for cystic fibrosis. Cochrane Database Syst Rev 2011 aug10:(8): CD002201. [PubMed]
These reviewers concluded that the data, from the 4 studies they found acceptable of 13 previous studies, suggested that regular omega-3 studies may provide some benefits for people with cystic fibrosis with relatively few adverse effects. However, the evidence was not adequate to recommend their routine use.
2013 Witters P. Dupont L. Vermeulen F. Proesmans M. Cassiman D. Wallemacq P. De Boeck K. Lung transplantation in cystic fibrosis normalizes essential fatty acid profiles. J Cyst Fibros 2023; 12:222-228. [PubMed]
Disorders in essential fatty acid state are increasingly reported and various supplementation trials have been performed in an attempt to improve outcomes. However, the mechanisms leading to these disturbances remain elusive. We wanted to investigate the role of the diseased CF lung on fatty acid profiles.
The authors compared fatty acid profiles in patients with CF after lung transplantation (n=11) to age-matched healthy controls and homozygous F508del patients (n=22 each). Compared to healthy controls, in patients with CF, there are decreased levels of docosahexaenoic, linoleic and arachidonic acid and increased levels of mead acid. In patients that underwent a lung transplantation, levels of docosahexaenoic, linoleic and arachidonic acid were normal. Mead acid did not decrease significantly.
The authors concluded that the diseased CFTR deficient lung is a major determinant in the disturbed fatty acid profile in CF.
2013 Alicandro G, Faelli N, Gagliardini R, Santini B, Magazzu G, Biffi A, Rise P, Galli C, Tirelli AS, Loi S, Valmarana L, Cirilli N, Palmas T, Vieni G, Bianchi ML, Agostoni C, Colombo C. A randomized placebo-controlled study on high-dose oral algal docosahexaenoic acid supplementation in children with cystic fibrosis. Prostag Leukotr Ess 2013; 88:163-169. [PubMed]
Low plasma concentrations of docosahexaenoic acid (DHA) are reported in unsupplemented cystic fibrosis (CF) patients. Forty-one CF patients aged from 6 to 12 years were randomized to receive high-dose DHA (100 mg/kg/day in the first month and 1g per day thereafter through a 12-month supplementation) or placebo (germ oil). Primary outcome was percentage change in plasma AA:DHA ratio. Secondary outcomes were changes in the number of pulmonary exacerbations compared to previous year, lung function, BMI, skinfold thicknesses, and body composition assessed by DXA and in serum concentrations of C-reactive protein, cytokines and vitamin (alpha-tocopherol and retinol).
Compared to the control group plasma AA:DHA ratio decreased in the intervention group after 6 months (median percentage changes: -73% in the intervention group vs. -10% in the control group, P=0.001). No differences were detected between groups for secondary outcomes.
Despite a decrease of the AA/DHA ratio, DHA supplementation for one year did not induce any significant biochemical and clinical improvement in CF patients. This is yet another study that failed to confirm the findings in mice of Beharry S et al. 2007. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol – Gastr L 2007; 292:G839-48.[PubMed].Beharry et al, 2007 for more discussion.
2013 Witters P. Dupont L. Vermeulen F. Proesmans M. Cassiman D. Wallemacq P. De Boeck K. Lung transplantation in cystic fibrosis normalizes essential fatty acid profiles. J Cyst Fibros 2013; 12:222-228. [PubMed]
Disorders in essential fatty acid state are increasingly reported and various supplementation trials have been performed in an attempt to improve outcomes. However, the mechanisms leading to these disturbances remain elusive. The authors investigated the role of the diseased CF lung on fatty acid profiles.
They compared fatty acid profiles in patients with CF after lung transplantation (n=11) to age-matched healthy controls and homozygous F508del patients (n=22 each). They found that compared to healthy controls, in patients with CF, there are decreased levels of docosahexaenoic, linoleic and arachidonic acid and increased levels of mead acid. In patients that underwent lung transplantation, levels of docosahexaenoic, linoleic and arachidonic acid were normal. Mead acid did not decrease significantly. The diseased CFTR deficient lung appeared to be a major determinant in the disturbed fatty acid profile in CF.
– This is new and interesting data as abnormal fatty acid profiles have been reported in CF since the first paper by Kuo et al in 1962 and there is still confusion regarding the cause and significance. There is a clear and helpful discussion on this difficult subject at the end of this present paper.
The authors conclude that “the CF lung is an important player in the development of abnormal fatty acid profile. Rather than studying the effect of fatty acids on lung function (as in EFA supplementation trial) we did the reverse by documenting fatty acid profiles in a subset of patients who underwent lung transplantation. We showed that post-transplantation an evolution toward normal fatty acid profiles is to be exptected”.
2016 Oliver C, Watson H. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst Rev. 2016 Jan 5;(1):CD002201. doi: 10.1002/14651858.CD002201.pub5.2673072
Studies suggest that a diet rich in omega-3 essential fatty acids may have beneficial anti-inflammatory effects for chronic conditions such as cystic fibrosis. This is an updated version of a previously published review. To determine whether there is evidence that omega-3 polyunsaturated fatty acid supplementation reduces morbidity and mortality and to identify any adverse events associated with supplementation. The searches identified 15 studies; four studies with 91 participants (children and adults) were included.
After reviewing these studies the authors concluded that regular omega-3 supplements may provide some benefits for people with cystic fibrosis with relatively few adverse effects, although evidence is insufficient to draw firm conclusions or recommend routine use of these supplements in people with cystic fibrosis. This review has highlighted the lack of data for many outcomes meaningful to people with or making treatment decisions about cystic fibrosis. A large, long-term, multicentre, randomised controlled study is needed to determine any significant therapeutic effect and to assess the influence of disease severity, dosage and duration of treatment. Future researchers should note the need for additional pancreatic enzymes.
– This conclusion sums up the situation regarding the use of omega-3 fatty acid supplements in cystic fibrosis.
Cottrill KA, Farinha CM, McCarty NA. The bidirectional relationship between CFTR and lipids. Commun Biol. 2020;3(1):179. Published 2020 Apr 20. doi:10.1038/s42003-020-0909-1 [Pubmed]

Kirsten Cottrill
While work to understand this CFTR has resulted in new treatment strategies, it is important to emphasise that CFTR exists within a complex lipid bilayer – a concept largely overlooked when performing structural and functional studies. In this review the authors discuss cellular lipid imbalances in CF, mechanisms by which lipids affect membrane protein activity, and the specific impact of detergents and lipids on CFTR function.
Kirsten A Cottrill is a Graduate Student at the McCarty Lab, Molecular and Systems Pharmacology Program, Emory University, Atlanta, GA, US
Alejandro López-Neyra Lucrecia Suárez, Marta Muñoz, Ana de Blas, Marta Ruiz de Valbuena, María Garriga, Joaquim Calvo, et al. Long-term docosahexaenoic acid (DHA) supplementation in cystic fibrosis patients: a randomized, multi-center, double-blind, placebo-controlled trial Prostaglandins Leukot Essent Fatty Acids 2020 Oct 1;162:102186.doi: 10.1016/j.plefa.2020.102186. Online ahead of print [Pubmed]

Alejandro Lopez Neyra
Cystic fibrosis (CF) patients have an alteration in fatty acid (FA) metabolism, associated with increased omega-6 and low omega-3 FA. Previous studies on supplementation with omega-3 FA in CF had contradictory results, and to date there is no evidence to recommend routine use of omega-3 supplements in CF patients. We hypothesized that long-term supplementation with docosahexaenoic acid (DHA) will have beneficial effects in these patients, by reducing pulmonary, systemic and intestinal inflammation.
Results – In this study, long-term DHA supplementation in CF patients was safe, but did not offer any benefit on inflammatory biomarkers, or in clinical outcomes compared with placebo.
Dr Alejanro Lopeez-Neyra is at the Unidad de Fibrosis Quística. Servicio de Pediatría. Hospital Universitario Ramón y Cajal. Cª Colmenar Km. 9,1. 28034-Madrid. Spain; Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS). Cª Colmenar Km. 9,1. 28034-Madrid. Spain.
Clarisse Vandebrouck, Thierry Ferreira Glued in lipids: Lipointoxication in cystic fibrosis. EBioMedicine 2020 Oct 7;61:103038.doi: 10.1016/j.ebiom.2020.103038.Online ahead of print. FREE [Pubmed]

Thierry Ferreira

Clarisse Vandebrouck
Cystic Fibrosis (CF) is an autosomal recessive disease caused by mutations in the CF transmembraneregulator (CFTR) gene, which encodes a chloride channel located at the apical surface of epithelial cells. Unsaturated Fatty Acid (UFA) deficiency has been a persistent observation in tissues from patients with CF. However, the impacts of such deficiencies on the etiology of the disease have been the object of intense debates. The aim of the present review is first to highlight the general consensus on fatty acid dysregulations that emerges from, sometimes apparently contradictory, studies. In a second step, a unifying mechanism for the potential impacts of these fatty acid dysregulations in CF cells, based on alterations of membrane biophysical properties (known as lipointoxication), is proposed. Finally, the contribution of lipointoxication to the progression of the CF disease and how it could affect the efficacy of current treatments is also discussed.
– The role of fatty acids has been a constantly recurring theme since the early Sixties long before CFTR was discovered and modulator treatments became available. This excellent review of the subject, in parts “heavy going” for the non-scientist, links the fatty acid abnormality with the CFTR function and the new treatments. The free full version is recommended.
Clarisse Vandebrouck from the Laboratoire “Lipointoxication and Channelopathies (LiTch) – ConicMeds”, Université de Poitiers, 1, rue Georges Bonnet, Poitiers, France; Laboratoire “Signalisation et Transports Ioniques Membranaires (STIM; EA 7349)”, Université de Poitiers, 1, rue Georges Bonnet, Poitiers, France.
Corresponding author Thierry Ferreira Director of Laboratories from the Laboratoire “Lipointoxication and Channelopathies (LiTch) – ConicMeds”, Université de Poitiers, 1, rue Georges Bonnet, Poitiers, France.
Deanne H Hryciw , Courtney A Jackson, Nirajan Shrestha, David Parsons, Martin Donnelley, Andrew J McAinch. Role for animal models in understanding essential fatty acid deficiency in cystic fibrosis. Cell Mol Life Sci. 2021 Nov 5.doi: 10.1007/s00018-021-04014-2.Online ahead of print. [Pubmed]

Deanne H Hryciw
Essential fatty acid deficiency has been observed in most patients with Cystic Fibrosis (CF); however, pancreatic supplementation does not restore the deficiency, suggesting a different pathology independent of the pancreas. At this time, the underlying pathological mechanisms are largely unknown. Essential fatty acids are obtained from the diet and processed by organs including the liver and intestine, two organs significantly impacted by mutations in the cystic fibrosis transmembrane conductance regulator gene (Cftr). There are several CF animal models in a variety of species that have been developed to investigate molecular mechanisms associated with the CF phenotype. Specifically, global and systemic mutations in Cftr which mimic genotypic changes identified in CF patients have been generated in mice, rats, sheep, pigs and ferrets. These mutations produce CFTR proteins with a gating defect, trafficking defect, or an absent or inactive CFTR channel. Essential fatty acids are critical to CFTR function, with a bidirectional relationship between CFTR and essential fatty acids proposed. Currently, there are limited analyses on the essential fatty acid status in most of these animal models. Of interest, in the mouse model, essential fatty acid status is dependent on the genotype and resultant phenotype of the mouse. Future investigations should identify an optimal animal model that has most of the phenotypic changes associated with CF including the essential fatty acid deficiencies, which can be used in the development of therapeutics.
Dr Deanne H Hryciw is a Senior Lecturer at the School of Environment and Science, Griffith University, Nathan, QLD, Australia. Centre for Planetary Health and Food Security, Griffith University, Nathan, QLD, Australia. Institute for Health and Sport, Victoria University, Melbourne, VIC, Australia.
Birgitta Strandvik. Nutrition in Cystic Fibrosis-Some Notes on the Fat Recommendations. Nutrients 2022 Feb 18;14(4):853.doi: 10.3390/nu14040853. Free PMC article [Pubmed]

Birgitta Strandvik
Nutrition is important in cystic fibrosis (CF) because the disease is associated with a higher energy consumption, special nutritional deficiencies, and malabsorption mainly related to pancreatic insufficiency. The clinical course with deterioration of lung function has been shown to relate to nutrition. Despite general recommendation of high energy intake, the clinical deterioration is difficult to restrain suggesting that special needs have not been identified and specified. It is well-known that the CF phenotype is associated with lipid abnormalities, especially in the essential or conditionally essential fatty acids.
This review will concentrate on the qualitative aspects of fat metabolism, which has mainly been neglected in dietary fat recommendations focusing on fat quantity. For more than 60 years it has been known and confirmed that the patients have a deficiency of linoleic acid, an n-6 essential fatty acid of importance for membrane structure and function. The ratio between arachidonic acid and docosahexaenoic acid, conditionally essential fatty acids of the n-6 and n-3 series, respectively, is often increased. The recently discovered relations between the CFTR modulators and lipid metabolism raise new interests in this field and together with new technology provide possibilities to specify further specify personalized therapy.
ProfessorBirgitta Strandvik is in the Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, 14183 Stockholm, Sweden.
-Professor Strandvik has been and remains a leading European authority on cystic fibrosis since the Sixties. This paper deals with one of her favourite areas, lipid metabolism in the era of CFTR modulators.
