LIVER and GALL BLADDER
1927 De Lange C. Cirrhosis of pancreas and liver in infant. Am J Dis Child 1927; 34:372-383.
Cornelia de Lange, famous for her description of the syndrome of multiple congenital anomalies which bears her name, described the third child of healthy parents who was jaundiced from the 7th day of life and admitted to hospital aged six weeks weighing only 2200 gm. The child appeared atrophic with peeling skin and hepatosplenomegaly and died two days later. At autopsy the pancreas showed a great increase in connective tissue and round cells but “excretory ducts showed nothing of importance”. Some islets were of abnormal size and sclerotic. The liver showed bile thrombi and evidence of obstruction. The heart and lungs were normal.
– It is difficult to accept that this infant definitely had CF, but there was neonatal obstructive jaundice and histological abnormalities of the pancreas and so CF would be a definite possibility.
1949 Pugsley HE, Spence PM. Case of cystic fibrosis of pancreas associated with chronic pulmonary disease and cirrhosis of the liver. Ann Int Med 1949; 30:1262-1272. [PubMed]
Cirrhosis of the liver in 17 year old white boy with CF and chronic pulmonary disease. At post mortem the liver was grossly nodular and microscopically the portal areas showed extensive irregular fibrosis with proliferation of the small bile ducts.
1953 Webster R, Williams H. Hepatic cirrhosis associated with fibrocystic disease of the pancreas: clinical and pathological reports of 5 patients. Arch Dis Child 1953; 28:343-350.[PubMed]
This is the first comprehensive paper on liver involvement in CF describing “an unusual type of multilobular portal cirrhosis”; three were identified at autopsy and two on liver biopsy. The authors considered that protein deficiency, due mainly to pancreatic dysfunction, was the cause of the liver damage.
However, di Sant’Agnese observed that CF was a generalised disease that could affect the liver as well as the pancreas, lungs and sweat glands. In commenting on this paper, he described six similar cases at the Babies Hospital New York – representing some two percent of the total CF clinic.
1955 Gatzimos CD, Jowitt RH. Jaundice in mucoviscidosis (fibrocystic disease of the pancreas). Report of four cases. Am J Dis Child 1955; 89:182. [PubMed]
In contrast to laboratory and pathological evidence of liver disease, clinical jaundice in people with CF is uncommon. From 1922 to 1954, 6741 autopsies were performed at Indianapolis University Medical Centre; 3517 were children. Fourteen had CF in four of whom jaundice had been the main complaint. The four of 14 with CF is a higher incidence of clinical jaundice than in previous series (Andersen, 1938 above; May & Lowe, 1949 above; Bodian, 1952 above). These authors suggested that CF should always be considered in the differential diagnosis of jaundice in infants.
– Subsequent experience has confirmed the advice that CF should always be excluded particularly when an infant with obstructive jaundice is suspected of having congenital biliary atresia, as on occasion an infant suspected of having congenital biliary atresia has been treated with a Kasai operation (a major procedure anastomosing the underside of the liver to the bowel in an attempt to overcome biliary atresia) only to discover later that the infant had CF with severe neonatal cholestatic jaundice (Perkins WG. et al. Cystic fibrosis mistaken for idiopathic biliary atresia. Clin Pediatr 1985; 24:107-109. [PubMed]).
1956 Blanc WA, di Sant’Agnese PA. A distinctive type of biliary cirrhosis of the liver associated with cystic fibrosis of the pancreas: recognition through signs of portal hypertension. Pediatrics 1956; 18: 387-409. [PubMed]
Bodian (1952 above) recognised that foci of biliary fibrosis occurred commonly as a necropsy finding.
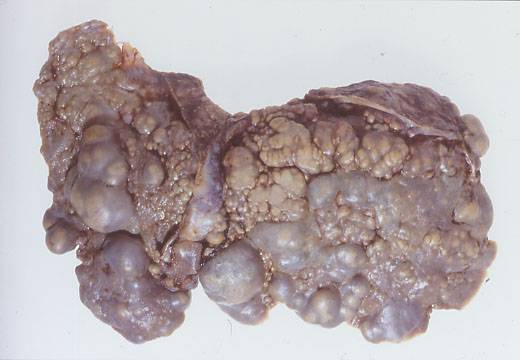
A liver removed from a 12 year old Leeds boy with CF who was in liver failure. He received a successful liver transplantation by the late Professor Geoff Giles at St James University Hospital Leeds around 1990.
Andersen (1938 above) reported three cases from the literature and one of her own where there was liver involvement. Here the progression of the early changes to a distinctive type of biliary cirrhosis (figure 1) accompanied by severe and at times fatal manifestations is reported.
Seven patients, aged four to 10 years, are reported; three died; four had raised portal pressures. The absence of jaundice was noted and the lack of consistency of “so-called” liver function tests. An infective or nutritional insult to an already abnormal liver was suggested. In these young patients the condition was first recognised by the appearance of signs of portal hypertension. The authors correctly predicted that “it is possible that symptoms due to failure of liver function may appear subsequently after prolonged survival”.(also Webster et al, 1953 above; Craig et al, 1957 below; Mieles et al, 1989 below).
1957 Craig J, Haddad M, Shwachman H. The pathological changes in the liver in cystic fibrosis of the pancreas. Am J Dis Child 1957; 93:357-369. [PubMed]
Description of the typical CF liver changes by Blanc and Craig (Blanc & di Sant’Agnese, 1956 above)
1957 Gibson JB, Rodgers HW. Portal hypertension in fibrocystic disease of the pancreas. Arch Dis Child 1957; 32:355-358. [PubMed]
Good results from removal of a large spleen which reached the level of the umbilicus and weighed 710 g in a boy aged nine years. The liver showed marked fibrosis and the authors considered the liver changes were not likely to be due solely to the pancreatic damage, but suggest that focal obstruction of the bile ductules was the prime lesion – an explanation which proved to be correct. They considered the main danger from liver disease in this condition was portal hypertension.
– Subsequent reports confirmed the benefit of splenectomy where the size of the organ was an embarrassment in terms of abdominal space even interfering with respiration and the descent of the diaphragm.
In 1988 a Leeds boy aged 16 years with CF had a massive spleen (3.2 kg) removed by the late Prof. Geoff Giles after which his FEV1 improved by 30% and he returned to normal sporting activities (Gilbert J, et al. Scand J Gastroenterol 1988; 23 Suppl 143:177).
1958 Jones MD, Sakai H, Rogerson AG. Cholangiography in children with fibrocystic disease of the pancreas; a pilot study. J Pediatr 1958; 53:172-179. [PubMed]
Seventeen children with CF (aged 19 months to 11 years) had intravenous Cholografin and the bile ducts were visualised in 12; in 13 the gallbladder was opacified.
1961 Alagille D, Vinh Le Tan. Hepatic localizations of mucoviscidosis. Tijdschr gastro-enterol 1961; 4:435-454.
The incidence of hepatic cirrhosis varies in different reports but all agree the complication increases with age. In 200 people with CF cirrhosis occurred in 1% aged less than 3 months, 6% between three and 12 months, 11% one to three years, 37% over three years of age. Histologically there was a focal biliary cirrhosis, diffuse multilobular cirrhosis and/or portal hypertension.
The lesson the authors took from their findings was that “Investigation of the young cirrhotic patient is incomplete without a sweat test” – which is still sound advice today.
1962 Esterly JR, Oppenheimer EH. Observations in cystic fibrosis of the pancreas. I. The gallbladder. Bull Johns Hopkins Hosp 1962; 110:247-55. [PubMed]
First description of gall bladder abnormalities noted at autopsy that were present in 24 of 72 people with cystic fibrosis. “The cystic duct may be atretic or stenotic from inspissated mucus or mucosal hyperplasia. Mucus distends the gallbladder epithelial cells and fills the lumen with a colourless secretion. The gallbladder may atrophy or persist as a thin walled cyst lined with flattened mucosa”. Radiological appearances of the gall bladder and bile ducts had been reported by Jones et al, 1958 (above).
1966 Wilroy RS Jr, Crawford SE, Johnson W. Cystic fibrosis with extensive fatty replacement of the liver. J Pediatr 1966; 68:67-73. [PubMed]
An early description of fatty infiltration of the liver (steatosis), which can result in gross hepatomegaly and is, in part, reversible when the intestinal malabsorption is treated and the nutritional state improved. The child reported in this paper presented at two years five months with an enlarged liver which “extended to the pelvic brim” and marked generalised oedema – no manifestations of pulmonary disease were noted at the time.
1971 Valman HB, France NE, Wallis PG. Prolonged jaundice in cystic fibrosis. Arch Dis Child 1971; 46: 805-809. [PubMed]
Bernard Valman reviewed previous reports of early hepatic changes (Bodian, 1952; di Sant’Agnese & Blanc, 1956 both below) and of neonatal obstructive jaundice in CF (Gatzimos & Jowitt, 1955; Bernstein et al, 1960; Shier & Horn, 1963; Kulczycki 1967; Talamo & Hendren, 1968). Four new infants with CF and jaundice were reported – resolving in two survivors and one that died of other causes. Histology already showed established cirrhosis in the infant who died at 22 weeks.
– This was an important paper showing a reasonably good prognosis for early prolonged obstructive jaundice in some CF infants. It is also important that this presentation of CF is recognised as infants with CF have been mistakenly thought to have biliary atresia only to be diagnosed as having CF after they had major surgery using a Kasai operation. In some of these CF infants the jaundice settles spontaneously over a few months; others have been reported to respond to treatment with ursodeoxycholic acid – the treatment introduced for older patients with liver disease in the early Nineties (Colombo et al, 1990 below).
1973 Weber A, Roy CC, Morin CL, Lasalle R. Malabsorption of bile acids in children with cystic fibrosis. New Engl J Med 1973; 289:1001. [PubMed]
Total faecal bile acid excretion had been reported to be increased (Leyland C. Arch Dis Child 1970; 45:714). This study of 26 children with CF aged two months to nine years from Professor Roy’s unit in Montreal confirmed that faecal bile acid levels may reach seven times the normal level.
1975 Feigelson J, Pecau Y, Cathelineau L, Navarro J. Additional data on hepatic function tests in cystic fibrosis. Acta Paediatr Scand 1975; 64:337-344. [PubMed]

Jean Feigelson
An early contribution (of many over the years) from Jean Feigelson of Paris who must have attended every CF meeting since they

Jean Navarro
started and still does! Since the early Sixties Jean Feigelson has published on a wide range of CF topics, usually based on long personal experience, but unfortunately often in French journals! In this paper he describes 9 of 50 patients with CF reported who had multilobular cirrhosis observed for 3 years.
The last author on this paper Dr Jean Navarro subsequently published many papers on CF, often in French, from Paris and became a leading researcher and clinician in both CF and paediatric gastroenterology and also in various roles in the French national CF organisation – Association Vaincre la Mucoviscidose.
1975 Goodchild MC, Murphy GM, Howell AM, Nutter SA, Anderson CM. Aspects of bile acid metabolism in cystic fibrosis. Arch Dis Child 1975; 50:769-777. [PubMed]
A detailed study by Mary Goodchild (who later moved to head the Cardiff Paediatric CF Unit) from Charlotte Anderson’s unit in Birmingham. Most children with CF had raised faecal bile acid levels which did not correlate with fat excretion but there was an inverse relationship with age. The authors suggested that chronic bile acid loss may eventually deplete the bile acid pool – which was in fact the case. Studies from Prof. Roy’s unit in Montreal also showed a greatly increased faecal loss of bile acids in children with cystic fibrosis (Weber et al, 1973; Roy et al, 1977 below).
1977 Roy CC, Weber AM, Morin CL, Combes JC, Nussle D, Megevand A, Lasalle R. Abnormal biliary lipid composition and cystic fibrosis. N Eng J Med 1977; 297:1301-1305. [PubMed]
Early description of the abnormal bile composition in CF from Prof. Roy’s unit in Montreal where there was a particular interest in paediatric liver disease. Cholesterol was similar to that of patients with cholelithiasis; also there were abnormal glycine/taurine ratios that improved with pancreatic enzymes (also Weber et al, 1973 above; Goodchild et al, 1975 above).
1977 Scott J, Elias E, Moult PJA, Barnes S, Wills MR. Rickets in adult cystic fibrosis with myopathy, pancreatic insufficiency and proximal renal tubular dysfunction. Am J Med 1977; 63:488-492. [PubMed]
One of the very few reports of rickets in a white man aged 19 years with CF in whom pancreatic and hepatic involvement was advanced. It was suggested that vitamin D malabsorption occurred due to gross bile salt deficiency due to the known increased bile acid loss in association with pancreatic steatorrhoea. There was evidence of secondary hyperparathyroidism with proximal renal tubular acidosis, aminoaciduria, phosphaturia and hypophosphatemia which may have accelerated the rachitic syndrome. Treatment with oral pancreatic and parenteral vitamin D supplements led to full recovery of the rachitic syndrome and the proximal renal tubular dysfunction.
1978 Schwarz HP, Kraemer R, Thurnheer U, Rossi E. Liver involvement in cystic fibrosis. A report of 9 cases. Helv Paediat Acta 1978; 33:351-364. [PubMed]
A report of 9 patients amongst the 204 seen at Professor Rossi’s clinic in Berne over the previous 20 years. Focal biliary cirrhosis was the main finding in three with non-specific nodular cirrhosis, an infant with prolonged obstructive neonatal jaundice and two others had steatosis. The authors review the previous literature.
1988 Gaskin KJ, Waters DL, Howman-Giles R, de Silva M, Earl JW, Martin HC, Kan AE, Brown JM, Dorney SF. Liver disease and common-bile-duct stenosis in cystic fibrosis. N Engl J Med 1988; 318:340-346. [PubMed]

Peter Cooper, Kevin Gaskin, Jim Littlewood, Peter Bye in Sydney 1990
This study from Sydney caused considerable interest. Hepatobiliary scans were performed on 50 of 61 patients with CF who had hepatomegaly, abnormal liver function, or both and also in 31 of 92 patients with CF who did not have hepatomegaly or abnormal liver function. Ninety-six percent of the patients with liver disease had evidence of biliary tract obstruction with a stricture of the distal common bile duct in the majority of cases. All the patients without liver disease had normal intra hepatic and common-duct excretion of tracer. The authors concluded that strictures of the distal common bile duct are common in patients with CF and liver disease. They said the association required further study, since surgical relief of common-duct obstruction may prevent or ameliorate the hepatic complications of cystic fibrosis.
– If strictures of the common bile duct were an important cause of CF related liver disease this was a very important observation. However, the results were questioned and the findings were contested in later publications (Nagel RA, Westaby D, Javaid A, et al. Liver disease and bile duct abnormalities in adults with cystic fibrosis. Lancet 1989; ii: 1422-1425.[PubMed]; Brien S, Keogan M, Caseu M, et al. Biliary complications of cystic fibrosis. Gut 1992; 33:387-391. [PubMed]) who concluded “these results indicate that abnormalities of the bile ducts in patients with cystic fibrosis related liver disease are confined to the intrahepatic biliary tree and that common bile duct strictures do not contribute to either the progression or development of liver disease in these patients”. Subsequently bile duct obstruction was not considered to be an important cause of CF related liver disease.
1989 Mieles LA, Orenstein D, Teperman L, Podesta L, Koneru B, Starzl TE. Liver transplant in cystic fibrosis. Lancet 1989; i: 1073. [PubMed]
Thomas Starzl
Liver transplantation has been used successfully in patients with CF and the results are surprisingly good. Lung function, far from deteriorating as a result of the long operation has improved in some patients (Noble-Jamieson et al, 1994). Successful heart-lung-liver transplantations (Noble-Jamieson et al, J R Soc Med 1996;89 (Suppl 27):31-37) and lung-liver transplantations have also been performed (Couetil et al, 1995; Couetil et al, 1997).
Figure shows the liver removed from a 12 year old boy in liver failure. This was the first CF patient who had a liver transplant in Leeds, by the late Professor Geoff Giles, soon after this first paper appeared from Starzl’s team in the United States. In fact we phoned the Starzl team and received helpful advice and encouragement before deciding on this transplant. The patient did very well after the operation.
1990 Colombo C, Battezzati PM, Crosignani A, Assaisso M, Ronchi M, Giunta A. Effects of taurine and ursodeoxycholic acid on liver function tests in patients with cystic fibrosis. Acta Universitatis Carolinae – Medica 1990; 36:148-151. [PubMed]
The report of a memorable presentation by Prof. Carla Colombo of Milan at the European CF Society meeting in Prague in

Carla Colombo
1989 which I was fortunate to hear. This was the first report of the beneficial effect of oral ursodeoxycholic acid (URSO) in people with CF associated liver disease – before this there was no treatment for the liver disease.
In nine CF patients with clinical and biochemical evidence of liver disease, taurine (30 mg/kg/day) was administered one month before and during the successive treatment with ursodeoxycholic acid (10-15 mg/kg/day). Standard liver function tests were determined before and after each period of treatment. Taurine administration produced only inconsistent changes of liver function tests from baseline, whereas after the addition of ursodeoxycholic acid there was a substantial improvement in all abnormal liver function tests (details reported in J Pediatr 1990; 117:482-489 below).
1990 Colombo C, Setchell KD, Podda M, Crosignani A, Roda A, Curcio L, Ronchi M, Giunta A. Effects of ursodeoxycholic acid therapy for liver disease associated with cystic fibrosis. J Pediatr 1990; 117:482- 489. [PubMed]
The published report from Milan of data presented in 1989 at the European CF Society Meeting in Prague (1990 above) reporting the favourable effect of ursodeoxycholic acid (URSO) treatment 10-15 mg /kg day in 9 patients with CF who had chronic liver disease (presumably the same patients as reported in Prague in 1989). Liver function tests improved significantly (AST- by 34%, alanine aminotransferase – by 41%, gamma gutamyl transpeptidase – by 41% and alkaline phosphatase– by 19%).
– Subsequently URSO was widely used in people with CF associated liver disease and represented one of the major advances in CF management. A further trail was published in 1996 (Colombo et al, 1996). Much information was published supporting URSO treatment at an early stage of liver involvement and eventually most people with evidence of liver involvement were treated with URSO.
However, a Cochrane Review updated in 2008/9 concluded “There is insufficient evidence to justify its (URSO) routine use in cystic fibrosis”. In this writer’s opinion this conclusion was unfortunate as it could influence clinicians to withhold treatment from people with liver involvement. The following statement would have been more appropriate – “There is a considerable amount of evidence that URSO improves liver function in people with CF particularly if used at an early stage; but, as yet, there is no clinical trial that conforms to Cochrane standards to show this”.
1991 Scott-Jupp R, Lama M, Tanner MS. Prevalence of liver disease in cystic fibrosis. Arch Dis Child 1991; 66:698-701. [PubMed]
A search for the presence of liver disease among 524 patients with CF in the UK by Dr Robert Scott-Jupp working with Prof. Stuart Tanner in Leicester; details of a further 576 patients were obtained from databases. The overall prevalence of overt liver disease as indicated by the presence of an enlarged liver or spleen (or both) was 4.2%. The age related prevalence rose to a peak in adolescence, and then fell in patients over 20 years old. The implied increase in mortality among those with liver disease was not explained by deaths from liver disease, which were rare. Male patients were significantly more affected than female, the ratio being 3:1 among adolescents. Increasing prevalence of liver disease in patients with cystic fibrosis was not just a result of longevity.
1996 Colombo C, Battezzi PM, Podda M, Bettinadi N, Giunta A. Ursodeoxycholic acid for liver disease associated with cystic fibrosis:a double blind multicenter trial. Hepatology 1996; 23:1484-1490. [PubMed]
A trial of ursodeoxycholic acid by Carla Colombo and colleagues from Milan who first reported the use in people with CF (Colombo et al, 1990 above). Fifty five patients from 12 CF centres over one year had either UDCA + taurine, UDCA + placebo, placebo + taurine or double placebo. The UDCA treated patients showed better clinical condition and improved biochemistry; also those treated with taurine had improved prealbumin levels.
Despite this trial, a Cochrane review, revised in 2008/9, considered “There is insufficient evidence to justify its (URSO) routine use in cystic fibrosis”. However, most clinicians consider there is clinical evidence and would disagree with the Cochrane Review and do use URSO at an early stage if there is any evidence of liver involvement.
1998 Lindblad A, Glaumann H, Strandvik B. A two-year prospective study of the effect of ursodeoxycholic acid on urinary bile acid excretion and liver morphology in cystic fibrosis-associated liver disease. Hepatology. 1998; 27:166-74. [PubMed]
The efficacy of 2 years of treatment with ursodeoxycholic acid (UDCA) in cystic fibrosis (CF)-associated liver disease was evaluated by liver biopsies and liver function tests in 10 patients aged 8 to 28 years. Blind evaluation of liver biopsies indicated improved liver morphology with less inflammation and/or bile duct proliferation than before treatment with UDCA in 7 patients. Only 1 patient had signs of progression of clinical liver disease. The secondary bile acids, such as lithocholic acid (LCA) and deoxycholic acid (DCA), did not increase significantly. The excretion pattern of glycosidic conjugates of UDCA and its metabolites was similar to that found in healthy individuals, UDCA and isoUDCA being mainly excreted in conjugation with N-acetylglucosamine.
This study shows that UDCA modulates inflammation in CF-associated liver disease and indicates improvement of liver morphology during 2 years of treatment.
1999 Sokol RJ, Durie PR. Recommendations for the management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr 1999; 28 Suppl 1:S1-13.[PubMed]
Recommendations of an expert group on management of CF related liver disease.
2002 Colombo C, Battezzati PM, Crosignani A, Morality A, Costantini D, Padoan R, Giunta A. Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology 202; 36:1374-1382. [PubMed]
Incidence of liver disease (LD) associated with cystic fibrosis (CF) and its clinical characterization still is unsettled. The authors assessed prospectively the incidence and risk factors of this complication, and its impact on the clinical course of CF. Between 1980 and 1990, they enrolled 177 CF patients without LD in a systematic clinical, laboratory, ultrasonography screening program of at least a 10-year duration. During a 14-year median follow-up (2,432 patient-years), 48 patients developed LD, with cirrhosis already present in 5. Incidence rate (number of cases per 100 patient-years) was 1.8% (95% confidence interval: 1.3-2.4), with sharp decline after the age of 10 years and higher risk in patients with a history of meconium ileus (incidence rate ratio, 5.5; 2.7-11), male sex (2.5; 1.3-4.9), or severe mutations (2.4; 1.2-4.8) at multivariate analysis.
Incidence of cirrhosis was 4.5% (2.3-7.8) during a median period of 5 years from diagnosis of liver disease. Among the 17 cirrhotic patients, 13 developed portal hypertension, 4 developed esophageal varices, 1 developed liver decompensation requiring liver transplantation. Development of LD did not condition different mortality (death rate ratio, 0.4; 0.1-1.5) or higher incidence of other clinically relevant outcomes.
In conclusion, LD is a relatively frequent and early complication of CF, whose detection should be focused at the first life decade in patients with history of meconium ileus, male sex, or severe genotype. Although LD does not condition a different clinical course of CF, in some patients it may progress rapidly and require liver transplantation.
– This was a major study from Carla Colombo, one of Europe’s leading authorities on CF liver disease.
2002 Williams SM, Goodman R, Thomson A, McHugh K, Lindsell DR. Ultrasound evaluation of liver disease in cystic fibrosis as part of an annual assessment clinic: a 9-year review. Clin Radiol 2002; 57:365-370. [PubMed].
To review 9 years of annual assessment data in cystic fibrosis (CF) and evaluate the frequency of hepatobiliary abnormalities and the correlation between ultrasound and biochemical findings. Over a 9-year period (1990-99), 168 children (age range 1-18 years) with CF have undergone an annual assessment which has included clinical, biochemical and ultrasonographic evaluation of the hepatobiliary system. We have retrospectively reviewed the sequential ultrasound reports and correlated them with the contemporaneous biochemical results. A total of 725 ultrasound examinations were performed over the review period. Sixty patients had at least one examination showing an abnormality of liver echo texture and in 39 patients this was a persisting finding. Seven patients (4.2%) developed frank cirrhotic change on ultrasound criteria, while 15 patients (8.9%) had evidence of persistent splenomegaly. Gall-bladder calculi were present in 4.8%. In 176 examinations (24%) there was disparity between the ultrasound findings and aspartate aminotransferase (AST) levels. In 3.0% of cases (five patients) there were persisting abnormalities of liver echo texture and persisting splenomegaly with a normal range AST value.
The authors concluded no perfect method of assessing hepatobiliary involvement in CF is currently available. Ultrasonographic and biochemical assessment may reflect different aspects of disease progression. Routine use of ultrasound in annual assessment allows identification of a minority of patients with liver changes but with normal biochemistry.
2003 Lenaerts C. Lapierre C. Patriquin H. Bureau N. Lepage G. Harel F. Marcotte J. Roy CC. Surveillance for cystic fibrosis-associated hepatobiliary disease: early ultrasound changes and predisposing factors. J Pediatr 2003; 143:343-350. [PubMed]
To investigate routine ultrasonography (US) as an early marker and to identify risk factors for the development of cirrhosis and portal hypertension (PHT) in cystic fibrosis (CF). 106 children with CF aged 5.9+/-2.3 years were followed for 10.4+/-0.2 years in a CF clinic. At enrollment, the US was normal, but biochemical and/or clinical disease was present in 10%. By the end of the study, 19 had developed US changes, eight with evidence of PHT. At the time of the initial US change, only 36.4% of those had, at the end of the study, either a heterogeneous or a nodular parenchyma, and only 50% of those with PHT had biochemical and/or clinical disease. Of the 30 patients treated with ursodeoxycholic acid for biochemical and/or clinical disease with (n=15) and without (n=15) associated US changes, PHT developed in six of the former and two of the latter. Univariate analysis and logistic regression showed that children with more severe disease in terms of forced expiratory volume in one second were at somewhat greater risk (P<.06) of PHT developing.
Ultrasound was an early marker of liver disease and more severe CF disease, a predictor of progressive liver disease. A controlled trial should be done to assess isolated US-detected disease as an indication for UDCA.
A record of experience and long follow up from a Montreal with extensive expertise in paedaitric liver disease
2003 Sheth S, Shea JC, Bishop MD, Chopra S, Regan MM, Malmberg E, Walker C, Ricci R, Tsui LC, Durie PR, Zielenski J, Freedman SD. Increased prevalence of CFTR mutations and variants and decreased chloride secretion in primary sclerosing cholangitis. Hum Genet 2003; 113:286-292. [PubMed]
This complex study concludes that the data indicate that there is an increased prevalence of CFTR abnormalities in PSC as demonstrated by molecular and functional analyses and that these abnormalities may contribute to the development of PSC in a subset of patients with inflammatory bowel disease.
2005 Fridell JA, Vianna R, Kwo PY, Howenstine M, Sannuti A, Molleston JP, Pescovitz MD, Tector AJ. Simultaneous liver and pancreas transplantation in patients with cystic fibrosis. Transplantation Proceedings 2005; 37:3567-9. [PubMed]
Liver transplantation is the treatment of choice for end stage cirrhosis in this setting, but the addition of an isolated simultaneous pancreas transplant in patients with CF related diabetes had not been reported. Two female patients with CF underwent simultaneous pancreas and liver transplantation. Both patients recovered well with normal liver function, resolution of portal hypertension, and normal blood glucoses independent of insulin. As a result of the enteric exocrine drainage of the pancreas, the patients now also independent of supplemental pancreatic enzymes.
Simultaneous liver and pancreas transplantation in CF patients provides the advantages of normalization of glucose and improved nutrition for patients requiring liver transplantation and should be considered in CF patients with CF related diabetes who require liver transplants. This is one way to deal with both liver failure and CF related diabetes which is such a major additional problem for adults with CF.
2006 Robberecht E, Van Biervliet S, Vanrentergem K, Kerremans I. Outcome of total splenectomy with port systemic shunt for massive splenomegaly and variceal bleeding in cystic fibrosis. Pediatr Surg 2006; 41:1561-5.[PubMed]
Six patients with CF (median age 14 years) underwent splenectomy with a splenorenal shunt operation – 3 for massive splenomegaly and three to control variceal bleeding after several sessions of sclerotherapy. Lung function remained stable, and there was an overall reduction of respiratory tract infections. The youngest patient, however, died of overwhelming septicemia during treatment with steroids.
Sometimes the spleen is so large in people with CF that the size is a physical handicap causing respiratory embarrassment and considerable abdominal distension in addition to the systemic effects of hypersplenism. A boy with CF of 15 years in Leeds had a spleen weighing over 3 kg removed by the late Prof Giles with great improvement in his respiratory function and general health (Gilbert J et al. Splenectomy for massive splenomegaly in cystic fibrosis with improvement in pulmonary status. Scand J Gastroenterol 1987; 23 (Suppl 143):177).
2006 Melzi ML, Kelly DA, Colombo C, Jara P, Manzanares J, Colledan M, Strazzabosco M, DeLorenzo P, Valsecchi MG, Adam R, Gridelli B, Assael BM, EGSLTCF, European Liver Transplant Association (ELTA), European Cystic Fibrosis Society (ECFS). Liver transplant in cystic fibrosis: a poll among European centers. A study from the European Liver Transplant Registry. Transplant International 2006; 19:726-731. [PubMed]
A report of 57 CF patients, many of whom were in their teens, who had liver transplants. Post-transplant survival was high and poor respiratory function was the main risk factor for early death; in the short-term, respiratory function significantly improved in most patients. Transplantation was considered to be the appropriate treatment for patients with advanced CF-related liver disease and preserved pulmonary function and an FEV1 over 50% predicted.
Further more general European experience of liver transplantation in people with CF confirming the good results since the first report of Mieles LA et al.(1989 above).
2007 Corno V, Dezza MC, Lucianetti A, Codazzi D, Carrara B, Pinelli D, Parigi PC, Guizzetti M, Strazzabosco M, Melzi ML, Gaffuri G, Sonzogni V, Rossi A, Fagiuoli S, Colledan M. Combined double lung-liver transplantation for cystic fibrosis without cardio-pulmonary by-pass. Am J Transpl 2007; 7:2433-2438. [PubMed]
The authors performed sequential bilateral single lung-liver transplantation – a therapeutic option for patients with end stage lung and liver disease. They performed the operation in three young men with cystic fibrosis. All the recipients had respiratory failure and portal hypertension with hypersplenism. The three recipients are alive with a median follow-up of 670 days (range 244-1,533). The operation is a complex but effective procedure for the treatment of end stage lung disease due to cystic fibrosis.
2007 Desmond CP, Wilson J, Bailey M, Clark D, Roberts SK. The benign course of liver disease in adults with cystic fibrosis and the effect of ursodeoxycholic acid. Liver Int 2007; 27:1402-1408. [PubMed]
The authors concluded that liver disease in 6.7% of 278 adults with CF is commonly complicated by portal hypertension (67%), but morbidity and mortality associated with this in their patient population were low. Ursodeoxycholic acid treatment was associated with improvement in hepatobiliary symptoms and liver function tests (50%).
This is a useful record of experience in a large adult CF unit; similar conclusions also came from experience at the adult CF centre in Cambridge UK (Nash et al, 2008 above).
2007 Pall H, Zielenski J, Jonas MM, DaSilva DA, Potvin KM, Yuan XW, Huang Q, Freedman SD. Primary sclerosing cholangitis in childhood is associated with abnormalities in cystic fibrosis-mediated chloride channel function. J Pediatr 2007; 151:255-259. [PubMed]
Twenty children with primary sclerosing cholangitis (PSC) diagnosed in childhood had sweat chloride concentration, nasal transmembrane potential difference, and extensive genetic analysis of the CFTR gene. Disease control subjects consisted of 14 patients with inflammatory bowel disease alone and no liver disease.
In the PSC group, CFTR chloride channel function was markedly diminished at -8.6 +/- 8.2 mV (reference range: -24.6 +/- 10.4 mV). In contrast, disease control subjects had normal function, at -17.8 +/- 9.7 mV (P = .008). Sweat chloride concentration in subjects with PSC was greater than in disease control subjects (20.8 +/- 3.4 mmol/L vs 12.0 +/- 1.6 mmol/L, P = .045). Comprehensive CFTR genotyping revealed that 5 of 19 (26.3%) subjects with PSC had a CFTR mutation or variant, compared with 6 of 14 (42.9%) disease control subjects. The authors concluded there is a high prevalence of CFTR-mediated ion transport dysfunction in subjects with childhood PSC.
The presence of one CF mutation in 26.3% of people with primary sclerosing cholangitis is similar to the increased frequency of CF mutations in pancreatitis. This is the first report of this association with sclerosing cholangitis. It is not clear why the CF mutations are so frequent in the control group and this is not explained.
2008 Nash KL, Allison ME, McKeon D, Lomas DJ, Haworth CS, Bilton D, Alexander GJ. A single centre experience of liver disease in adults with cystic fibrosis 1995-2006. J Cyst Fibros 2008; 7:252-257. [PubMed]
Liver disease is an important cause of death in adults with cystic fibrosis. Ursodeoxycholic acid (UDCA) may slow progression. Managing varices and timely evaluation for liver transplantation are important. 154 patients attending the CF Centre at Papworth, Cambridge in the UK were followed for a median 5 years. 43 had significant liver disease. Only one patient developed chronic liver failure and none required liver transplantation. 27 underwent endoscopy; 1 required variceal banding, the others had insignificant varices. Ultrasound was normal in 97 patients while five had steatosis; nine further patients had splenomegaly but no other evidence of portal hypertension. Neither spleen size nor platelet count correlated with portal hypertension. So liver disease was common in adults with CF but disease progression was rare.
Thus liver disease detected and closely monitored in adults appeared to have a milder course than in childhood CF. Splenomegaly, unrelated to portal hypertension may be a consequence of the cystic fibrosis.
2008 Mueller-Abt PR, Frawley KJ, Greer RM, Lewindon PJ. Comparison of ultrasound and biopsy findings in children with cystic fibrosis related liver disease. J Cyst Fibros 2008; 7:215-221. [PubMed].
The objective of this study from Brisbane was to determine if hepatic ultrasound findings in paediatric patients with cystic fibrosis and suspected liver disease are related to histopathological results derived from liver biopsies.
The authors concluded that the diagnosis of early liver disease in cystic fibrosis cannot reliably be made on the basis of ultrasound alone. A normal ultrasound does not preclude significant liver fibrosis in cystic fibrosis. An abnormal US that suggests cirrhosis predicts the presence of moderate to severe liver disease.
2009 Bartlett JR, Friedman KJ, Ling SC, et al. Gene Modifier Study Group. Genetic modifiers of liver disease in cystic fibrosis. JAMA 2009; 302:1076-1083. [PubMed]
A subset (approximately 3%-5%) of patients with CF develops severe liver disease with portal hypertension. The objective of the study was to assess whether any of 9 polymorphisms in 5 candidate genes (alpha(1)-antitrypsin or alpha(1)-antiprotease [SERPINA1], angiotensin-converting enzyme [ACE], glutathione S-transferase [GSTP1], mannose-binding lectin 2 [MBL2], and transforming growth factor beta1 [TGFB1]) are associated with severe liver disease in patients with CF.
The authors concluded that the SERPINA1 Z allele is a risk factor for liver disease in CF. Patients who carry the Z allele are at greater risk (OR, approximately 5) of developing severe liver disease with portal hypertension.
2009 Perera E, Massie J, Phillips RJ. Treatment of acne with oral isotretinoin in patients with cystic fibrosis. Arch Dis Child 2009; 94:583-586.[PubMed]
Theoretical concerns about liver disease and vitamin A deficiency have limited the use of oral isotretinoin for troublesome acne in adolescents with cystic fibrosis. Oral isotretinoin was administered to nine patients with cystic fibrosis who had troublesome acne unresponsive to antibiotics. All patients were followed for 1-4 years after cessation of treatment. Isotretinoin treatment cleared active acne lesions in all patients. It was well tolerated, and no patient had significant side effects. All nine patients were pleased or delighted with the improvement in their skin. Adolescents with cystic fibrosis and acne can be treated with oral isotretinoin. Oral isotretinoin should be considered for adolescents with cystic fibrosis who have acne associated with scarring, acne not clearing with topical and antibiotic treatment, acne associated with depression or severe cystic acne.
This is a helpful paper for those considering the use of isotretinoin but who may have reservations regarding liver toxicity.
2011 Lewindon PJ, Shepherd RW, Walsh MJ, Greer RM, Williamson R, Pereira TN, Frawley K, Bell SC, Smith JL, Ramm GA. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology 2011; 53:193-201. [PubMed]
Forty patients with cystic fibrosis (median age = 10.6 years) with abnormal clinical, biochemical, and US findings were subjected to dual-pass percutaneous liver biopsy. Clinical outcomes were recorded over 12 years of follow-up (median = 9.5 years for survivors). Logistic regression and receiver operating characteristic analyses were applied to predict hepatic fibrosis (which was assessed by fibrosis staging and quantitative immunohistochemistry) and the occurrence of PHT. PHT occurred in 17 of 40 patients (42%), including 6 of 7 (17%) who died during follow-up. Clinical examination, serum ALT levels, and US findings failed to predict either the presence of liver fibrosis or the development of portal hypertension (PHT). Fibrosis staging on liver biopsy, where the accuracy was improved by dual passes (P = 0.002, nonconcordance = 38%), predicted the development of PHT (P < 0.001), which occurred more frequently and at a younger age in those with severe fibrosis.
The authors conclude that although clinical modalities currently employed to evaluate suspected CFLD help to identify a cohort of children at risk for liver disease and adverse outcomes but do not predict an individual’s risk of liver fibrosis or PHT development. Liver fibrosis on biopsy predicts the development of clinically significant liver disease. Dual passes help to address sampling concerns. Liver biopsy has a relevant role in the management of patients with suspected CFLD and deserves more widespread application.
The following three papers (*) should be read in sequence as they concern the possibilty of UDCA toxicity. They are first the ECFS Best Pactice Guidelines for CF Related Liver Disease, then a letter commenting on the Guidelines followed by a response from the authors of the ECFS Guidelines
*2011 Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. [Review] J Cyst Fibros 2011; 10 Suppl 2:S29-36. [PubMed] [Full version available on the ECFS website}
These are the European guidelines for the management of CF liver disease and are available on the ECFS website.
Approximately 5-10% of cystic fibrosis (CF) patients develop multilobular cirrhosis during the first decade of life. Most CF patients later develop signs of portal hypertension with complications, mainly variceal bleeding. Liver failure usually occurs later, after the paediatric age. Annual screening for liver disease is recommended to detect pre-symptomatic signs and initiate ursodeoxycholic acid therapy, which might halt disease progression. Liver disease should be considered if at least two of the following variables are present: abnormal physical examination, persistently abnormal liver function tests and pathological ultrasonography. If there is diagnostic doubt, a liver biopsy is indicated. All CF patients with liver disease need annual follow-up to evaluate the development of cirrhosis, portal hypertension or liver failure. Management should focus on nutrition, prevention of bleeding and variceal decompression. UDCA should be started as soon as a diagnosis if CF related liver disease is made. Deterioration of pulmonary function is an important consideration for liver transplantation, particularly in children with hepatic dysfunction or advanced portal hypertension.
See letter of comment from Dr Ooi CY et al. 2012 below.
2012 Ooi CY, Nightingale S, Durie PR, Freedman SD. J Cyst Fibros 2012; 11:72-73 Ursodeoxycholic acid in cystic fibrosis-associated liver disease.[PubMed]
This is a letter commenting on the Debray et al 2011 Best practice guidelines (above). Essentially the authors are concerned about the possible dangers of UDCA in view of the adverse effects reported in the 5 year trial of the drug in adults with primary sclerosing cholangitis (Lindor KD et al. 2009.[PubMed]) where the treated patients, although having improved liver function tests, had a significantly higher incidence of major adverse effects (death and liver transplantation) and no better survival. They state “Simply put at present there are no convincing data to demonstrate that UDCA is efficacious or harmful in CF related liver disease”.
Reply below from Carla Colombo and colleagues
*2012 Colombo C, Debray D, Kelly D, Houwen R, Battezzati PM, Strandvik. Response to the letter by Ooi et al 2012. J Cyst Fibros 2012; 11:74-75.
The authors of the Debray et al Best Practice Guidelines note that the concerns of Ooi et al are based mainly on the study of Lindor et al 2009 and they did not regard it pertinent to compare CFRL with PSC as they are of different aetiology, pathology, degree of cholestasis and prognosis. They do mention that UDCA can be epimerized to chenodeoxycholic acid and also transformed by bacterial flora to lithocholic acid which are both liver toxic. The higher doses used by Lindor et al may have increased the possibility of this transformation but they did not investigate the bile acid pattern. Some of the European group are currently investigating the fate of this bile acid using mass spectrometry with stable-isotope dilution analysis.
They emphasise the lack of clinical ill effects after 20 years experience, request they be informed of any such ill effects but meanwhile state “we deem there are no compelling arguments for precluding CF patients to start UDCA early, before the liver disease has potentially progressed to a stage at which changing the course by any medical treatment is no longer possible”.
2012 Dowman JK, Watson D, Loganathan S, Gunson BK, Hodson J, Mirza DF, Clarke J, Lloyd C, Honeybourne D, Whitehouse JL, Nash EF, Kelly D, van Mourik I, Newsome PN. Long-term impact of liver transplantation on respiratory function and nutritional status in children and adults with cystic fibrosis. Am J Transplant 2012; 12:954-964. [PubMed]
Early liver transplant (LT) has been advocated for patients with cystic fibrosis liver disease (CFLD) and evidence of deterioration in nutritional state and respiratory function to prevent further decline. However, the impact of single LT on long-term respiratory function and nutritional status has not been adequately addressed. We performed a retrospective analysis of the outcomes of 40 (21 adult/19 pediatric) patients with CFLD transplanted between 1987 and 2009 with median follow-up of 47.8 months (range 4-180). One and five-year actuarial survival rates were 85%/64% for adult and 90%/85% for pediatric LT cohorts, respectively. Lung function remained stable until 4 years (FEV(1) % predicted; pretransplant 48.4% vs. 45.9%, 4 years post transplant) but declined by 5 years (42.4%). Up to 4 years post transplant mean annual decline in FEV(1) % was lower (0.74%; p = 0.04) compared with the predicted 3% annual decline in CF patients with co-morbidities including diabetes. Number of courses of intravenous antibiotics was reduced following LT, from 3.9/year pre transplant to 1.1/year, 5 years post transplant. Body mass index was preserved post transplant; 18.0 kg/m(2) (range 15-24.3) pre transplant versus 19.6 kg/m(2) (range 16.4-22.7) 5 years post transplant.
In conclusion, liver transplantation is an effective treatment for selected patients with cirrhosis due to CFLD, stabilizing aspects of long-term lung function and preserving nutritional status.
A substantial number of these patients did well after liver transplantation with good survival rates, stable lung function and a slowing of FEV1 decline. Also there were nutritional benefits. Although clinicians have observed such benefits in individual patients since the first liver transplants in people with CF were reported (Mieles LA et al. Lancet 1989:i:1073. above) it is good to have the benefits of such a major procedure confirmed in a relatively large series.
2011 Lewindon PJ, Shepherd RW, Walsh MJ, Greer RM, Williamson R, Pereira TN, Frawley K, Bell SC, Smith JL, Ramm GA. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology 2011; 53:193-201. [PubMed]
Cystic fibrosis liver disease (CFLD), which results from progressive hepatobiliary fibrosis, is an important cause of morbidity and mortality, but it is difficult to identify before portal hypertension (PHT) ensues. Clinical signs, serum alanine aminotransferase (ALT) levels, and ultrasound (US) are widely applied, but their value in predicting the presence of cirrhosis, the development of PHT, or adverse outcomes is undetermined. The potential gold standard, liver biopsy, is not standard practice and, notwithstanding sampling error considerations, has not been systematically evaluated. Forty patients with cystic fibrosis (median age = 10.6 years) with abnormal clinical, biochemical, and US findings were subjected to dual-pass percutaneous liver biopsy. Clinical outcomes were recorded over 12 years of follow-up (median = 9.5 years for survivors). Logistic regression and receiver operating characteristic analyses were applied to predict hepatic fibrosis (which was assessed by fibrosis staging and quantitative immunohistochemistry) and the occurrence of PHT. PHT occurred in 17 of 40 patients (42%), including 6 of 7 (17%) who died during follow-up. Clinical examination, serum ALT levels, and US findings failed to predict either the presence of liver fibrosis or the development of PHT. Fibrosis staging on liver biopsy, where the accuracy was improved by dual passes (P = 0.002, nonconcordance = 38%), predicted the development of PHT (P < 0.001), which occurred more frequently and at a younger age in those with severe fibrosis.
The authors conclude that although clinical modalities currently employed to evaluate suspected CFLD help to identify a cohort of children at risk for liver disease and adverse outcomes but do not predict an individual’s risk of liver fibrosis or PHT development. Liver fibrosis on biopsy predicts the development of clinically significant liver disease. Dual passes help to address sampling concerns. Liver biopsy has a relevant role in the management of patients with suspected CFLD and deserves more widespread application.
2012 Kappler M, Espach C, Schweiger-Kabesch A, Lang T, Hartl D, Hector A, Glasmacher C, Griese M. Ursodeoxycholic acid therapy in cystic fibrosis liver disease–a retrospective long-term follow-up case-control study. Aliment Pharmacol Ther 2012; 36:266-273.[PubMed]
To assess the long-term effects of continuous ursodeoxycholic acid (UDCA) therapy in cystic fibrosis patients with constantly elevated serum liver enzymes.The primary endpoint was the incidence of overt liver disease. Between 1989 and 2005, UDCA treatment was started in 98 subjects from a cohort of 382 cystic fibrosis patients. These subjects were compared with a historic control group of 352 subjects who attended our centre between 1975 and 1989 before UDCA became standard treatment. For the long-term comparison of liver function and lung function tests, a group of 98 matched contemporary cystic fibrosis patients were compared with the 98 subjects treated with UDCA.
Overt liver disease developed in only one of the 382 patients who was treated with UDCA for increased serum liver enzymes compared with nine patients in the historic control group (P < 0.05). Serum liver enzyme levels declined in most patients receiving UDCA treatment during the 17-year follow-up (87/98, P < 0.05). No difference was seen in lung function between subjects with cystic fibrosis-related liver disease and the matched controls.
The authors concluded that regular and systematic screening for liver involvement enables early introduction of UDCA therapy in affected cystic fibrosis patients, reduces the development of severe liver disease and leads to a significant and persistent improvement in serum liver tests, without impairing long-term pulmonary outcome.
– This is an interesting and reassuring record of experience in the use of UDCA over time in a large CF Centre in view of the concerns of Oii et al, 2012 above.
2014 Bruns F. Bremer M. Dettmer A. Janssen S. Low-dose splenic irradiation in symptomatic congestive splenomegaly: report of five cases with literature data. [Review] Oncology. 9:86, 2014.[PubMed]
Five patients (only 1 with CF) with symptomatic congestive splenomegaly received splenic irradiation with a total dose of 3 Gy (single dose: 0.5 Gy). One patient was re-irradiated after long-term failure with the same treatment schedule. Four patients obtained long term relief of splenic pain during the follow-up time of median 20 (range: 2-36) months. Four patients showed haematological response after irradiation with an increase of erythrocytes, leucocytes and/or platelets. A slightly decrease in spleen size was found in two patients.
The authors concluded low-dose splenic irradiation in symptomatic congestive splenomegaly is feasible and effective.
Splenic size in itself sometimes becomes a physical embarrassment to respiration and the space in the abdominal cavity. In such cases splenectomy has proved beneficial – radiation is obviously an alternative particularly if major surgery were to be contraindicated by the patient’s general condition.
Rowland M, Gallagher C, Gallagher CG, Laoide RÓ, Canny G, Broderick AM, Drummond J, Greally P, Slattery D, Daly L, McElvaney NG, Bourke B. Outcome in patients with cystic fibrosis liver disease. J Cyst Fibros. 2015 Jan;14(1):120-6. doi: 10.1016/j.jcf.2014.05.013. Epub 2014 Jun 7 [PubMed]
Irish children with CF liver disease (CFLD), and their age and gender matched controls were enrolled at baseline and reviewed after 10 years to determine which characteristics predict mortality.
72/84 (85.71%) of participants were followed, (mean age Cases 21.71yrs SD 6.5, CF controls 23.62 SD 5.6, 22 (61%) males), with no difference in duration of follow-up. Nineteen participants (26.4%) died,
38.9% (14/36) with CFLD and 13.89% (5/36) CF controls (Odds Ratio (OR) 3.94 95% CI:1.23-12.56 p=0.005).
In logistic regression, liver disease (OR 4.28 95% CI 1.07-17.16) female gender (OR 12.25 95% CI 2.37-63.24), reduced pulmonary function, (OR 5.11 95% CI 1.09-23.81) were each independent risk factors for mortality in CF. The authors concluded liver disease is an independent risk factor for mortality in CF.
Colombo C, Crosignani A, Alicandro G, Zhang W, Biffi A, Motta V, Corti F, Setchell KD. Long-Term Ursodeoxycholic Acid Therapy Does Not Alter Lithocholic Acid Levels in Patients with Cystic Fibrosis with Associated Liver Disease. J Pediatr. 2016 Jun 10. pii: S0022-3476(16)30216-5. doi: 10.1016/j.jpeds.2016.05.008. [Epub ahead of print]. [PubMed]
To evaluate the fasting and postprandial serum bile acid composition in patients with cystic fibrosis-associated liver disease (CFLD) after chronic administration of ursodeoxycholic acid (UDCA) (20mg/kg/day). The aim was to specifically focus on the extent of biotransformation of UDCA to its hepatotoxic metabolite, lithocholic acid, because of recent concerns regarding the safety of long-term, high-dose UDCA treatment for CFLD.Twenty patients with CFLD (median age 16years, range: 2.4-35.0) prescribed UDCA therapy for at least 2years were studied. Total and individual serum bile acids were measured by stable-isotope dilution mass spectrometry, in fasting and 2-hour postprandial samples taken during chronic UDCA (20mg/kg/day) administration.During chronic UDCA administration (median duration 8years, IQR: 6-16), UDCA became the predominant serum bile acid in all patients (median, IQR: 3.17, 1.25-5.56μmol/L) and chenodeoxycholic acid concentrations were greater than cholic acid (1.86, 1.00-4.70μmol/L vs 0.40, 0.24-2.71μmol/L). The secondary bile acids, deoxycholate and lithocholate, were present in very low concentrations in fasted serum (<0.05μmol/L). After UDCA administration, 2-hour postprandial concentrations of both UDCA and chenodeoxycholic acid significantly increased (P<.01), but no significant changes in serum lithocholic acid concentrations were observed.
– These data do not support recent suggestions that enhanced biotransformation of UDCA to the hepatotoxic secondary bile acid lithocholic occurs when patients with CFLD are treated with relatively high doses of UDCA.
Carla Colombo is Professor of Paediatrics in Milan and has pioneered the use of URSO for people with cystic fibrosis since 1990.
Drzymala-Czyz S, Jończyk-Potoczna K, Lisowska A, Stajgis M, Walkowiak J. Supplementation of ursodeoxycholic acid improves fat digestion and absorption in cystic fibrosis patients with mild liver involvement. Eur J Gastroenterol Hepatol. 2016 Feb 12. [Epub ahead of print][PubMed] Ursodeoxycholic acid (UDCA) supplementation is recommended for cystic fibrosis (CF) patients with associated liver disease. However, its effect on fat digestion and absorption is not known.In 23 patients with mild liver involvement, a C-mixed triglyceride breath test was performed on UDCA supplementation (with and without pancreatic enzymes – standard and increased dose) and after 1 month of UDCA withdrawal. Cumulative percentage dose recovery [CPDR; median (interquartile range)] has been considered to reflect lipid digestion and absorption.The enzyme supplementation resulted in a significant CPDR improvement [0% (0-0) vs. 4.6% (0.4-6.0); P<0.00046]. With the increased dose of enzymes in 16 patients with abnormal C-mixed triglyceride breath test results and lipase dose less than 3000 U/g of fat, higher CPDR values [8.6% (5.6-12.7); P<0.000027] were observed. However, a 1-month UDCA withdrawal resulted in a significant reduction in (P<0.000031) fat digestion and absorption [2.9% (0.7-5.8)].
The authors conclude that UDCA supplementation seems to enhance lipid digestion and absorption in pancreatic insufficient CF patients with mild liver involvement. This finding points toward the potential impact of UDCA supplementation on nutritional status in CF patients with liver disease and underscores the often-overlooked role of factors other than pancreatic enzymes on digestion and absorption of fats in CF.
2016 Stonebraker JR, Ooi CY, Pace RG, Corvol H, Knowles MR, Durie PR, Ling SC. Features of Severe Liver Disease With Portal Hypertension in Patients with Cystic Fibrosis. Clin Gastroenterol Hepatol. 2016 Apr 5. pii: S1542-3565(16)30016-7. doi: 10.1016/j.cgh.2016.03.041. [Epub ahead of print] [PubMed] A retrospective analysis of data from 561 patients with CF (63% male, 99% with pancreatic insufficiency), liver disease. All patients were enrolled in the Genetic Modifier Study of Severe CF Liver Disease at 76 international centers, from January 1999 through July 2013.Male patients were diagnosed with liver disease at a younger age than female patients (10 vs 11 years; P=.01). Splenomegaly was observed in 99% of patients and varices in 71%. Levels of liver enzymes were near normal in most patients. Thrombocytopenia affected 70% of patients and was more severe in patients with varices (88×109/L vs. 145×109/L; P<.0001). Ninety-one patients received liver transplants (16%), at a median age of 13.9 years. Compared to patients who did not receive liver transplants, patients who received liver transplants had lower platelet counts (78×109/L vs 113×100/L; P<.0001), higher international normalized ratios (P<.0001), and lower levels of albumin (P=.0002). The aminotransferase to platelet ratio index (APRI) and fibrosis index based on 4 factor (FIB4) values were above diagnostic thresholds for CF liver disease in 96% and 90% of patients, respectively. Patients who received liver transplants or who had varices had higher APRI and FIB4 values than patients who did not.In patients with CF, severe liver disease develops early in childhood (around 10 years of age) and is more common in boys than girls. Patients with varices and those who receive liver transplants have high platelet counts and APRI and FIB4 scores.
– A large series of patients the findings confirming, in particular, the fact that liver disease tends to develop early in childhood.
2016 van der Feen C; van der Doef HP; van der Ent CK; Houwen RH. Ursodeoxycholic acid treatment is associated with improvement of liver stiffness in cystic fibrosis patients. J Cyst Fibros 2016 Jul 29. pii: S1569-1993(16)30566-5. doi: 10.1016/j.jcf.2016.07.009. [PubMed]
Ursodeoxycholic acid (UDCA) might prevent progression of cystic fibrosis liver disease, but objective parameters for its effect are lacking. The authors used liver stiffness measurements to evaluate the effect of ursodeoxycholic acid. Paired measurements of liver stiffness were done in 73 patients without UDCA and in 32 patients with UDCA. In the latter group, 6 patients had cirrhosis; in 15 patients, UDCA was started based on Colombo criteria, and in 11 patients for other reasons. In patients without UDCA, liver stiffness increased: 0.19 (-0.03 to 0.59) kPa/year. Liver stiffness also increased in patients with cirrhosis: 4.6 (0.67-12.4)kPa/year. In patients who had UDCA based on Colombo criteria, a decrease of liver stiffness was observed: 0.70 (-1.6 to 0.55)kPa/year (P=0.01). In patients on UDCA for other reasons, liver stiffness increased: 0.23 (-0.20 to 0.51)kPa/year.
The authors concluded UDCA reduced liver stiffness in patients with well-defined, mild liver disease.They noted that these data were the first objective evidence of the beneficial effect if UDCA therapy in this group of patients with mild liver involvement. They suggest that UDCA started before severe liver damage is present, might be able to prevent progression of CF related liver disease and may even induce a reversal of fibrosis
[*Carla Colombo’s criteria for URSO treatment in CF – At least two of the following criteria – hepatomegaly confirmed by ultrasound, other U/S abnormalities of liver parenchyma and persistently increased liver enzymes (ASAT, ALAT, GGT) – being abnormal for at least 12 months].
Dr Catherine van der Feen is a paediatrician at Jeroen Bosch Ziekenhuis in the Netherlands
2016 Woodruff SA; Sontag MK; Accurso FJ; Sokol RJ; Narkewicz MR. Prevalence of elevated liver enzymes in children with cystic fibrosis diagnosed by newborn screen. J Cyst Fibros 2016 Aug 20. [PubMed
Prevalence and risks for elevated liver enzymes have not been studied systematically in children with CF identified by newborn screening by the team in Denver. 298 CF children had been identified by newborn screen since 1982. AST, ALT and GGT were tested at annual visits. The percent of children with 1 or >2 values of elevated AST, ALT and GGT determined. Relationship of liver enzymes to clinical factors or subsequent liver disease was analyzedAt least one abnormal value for AST (63%), ALT (93%) and ALT >1.5x ULN (52%) occurred by 21years of age. Liver enzyme elevations were not correlated with CFTR mutation, meconium ileus or ethnicity. AST and GGT >1.5x ULN were associated with later advanced liver disease HR (CI) 6.53 (2.02-21.1) and 4.03 (1.15-13.45), respectively.The authors concluded elevated liver enzymes are common during childhood in CF patients identified by newborn screening. Elevated AST and GGT may be markers for risk of advanced liver disease.
– A useful long-term study from Colorado, one of the first areas to introduce newborn CF screening in 1982 where Keith Hammond was the involved biochemist. The opportunity to undertake long-term follow-up, identifying findings that may indicate later significant liver involvement, is helpful. The finding raised AST, GGT would perhaps influence the earlier introduction of treatment with ursodeoxycholic acid?
2017 Cheng K, Ashby D, Smyth RL. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst Rev. 2017 Sep 11;9:CD000222. doi:10.1002/14651858.CD000222.pub4. [Epub ahead of print] [Pubmed]
To analyse evidence that ursodeoxycholic acid improves indices of liver function, reduces the risk of developing chronic liver disease and improves outcomes. Twelve trials have been identified, of which four trials involving 137 participants were included; data were only available from three of the trials (118 participants) since one cross-over trial did not report appropriate data. The dose of ursodeoxycholic acid ranged from 10 to 20 mg/kg/day for up to 12 months. The complex design used in two trials meant that data could only be analysed for subsets of participants. There was no significant difference in weight change, mean difference -0.90 kg (95% confidence interval -1.94 to 0.14) based on 30 participants from two trials. Improvement in biliary excretion was reported in only one trial and no significant change after treatment was shown. There were no data available for analysis for long-term outcomes such as death or need for liver transplantation.
The authors of the review concluded there were few trials assessing the effectiveness of ursodeoxycholic acid. The quality of the evidence identified ranged from low to very low. They consider there is currently insufficient evidence to justify its routine use in cystic fibrosis.
– Despite the conclusions of these Cochrane Reviewers, most experienced clinicians, including this writer when he was practising, would treat people with CF and early signs of liver abnormality with regular URSO. Before the introduction of URSO for children with CF by Carla Colombo in 1989, there was nothing one could do to halt the progression of liver involvement, which in some children had a serious outcome.
The latest text book advice (Cystic Fibrosis 4th Ed – Bush, Bilton & Hodson, 2016) on the use of URSO states that “it is likely that this less than objective use of UDCA in patients with CF liver disease will continue based on the evidence that is available and in the setting of a drug that is well tolerated and has very few associated adverse effects”.
2017 Colombo C; Crosignani A; Alicandro G; Zhang W; Biffi A; Motta V; Corti F; Setchell KD. Long-Term Ursodeoxycholic Acid Therapy Does Not Alter Lithocholic Acid. Levels in Patients with Cystic Fibrosis with Associated Liver Disease. J Pediatr 2016; 177:59-65.[Pubmed]
To evaluate the fasting and postprandial serum bile acid composition in patients with cystic fibrosis-associated liver disease (CFLD) after chronic administration of ursodeoxycholic acid (UDCA) (20mg/kg/day). The aim was to specifically focus on the extent of biotransformation of UDCA to its hepatotoxic metabolite, lithocholic acid because of recent concerns regarding the safety of long-term, high-dose UDCA treatment for CFLD. During chronic UDCA administration (median duration 8 years, IQR: 6-16), UDCA became the predominant serum bile acid in all patient (median, IQR: 3.17, 1.25-5.56 mumol/L) and chenodeoxycholic acid concentrations were greater than cholic acid (1.86, 1.00-4.70 mumol/L vs 0.40, 0.24-2.71 mumol/L). The secondary bile acids, deoxycholate and lithocholate, were present in very low concentrations in fasted serum (<0.05 mumol/L). After UDCA administration, 2-hour postprandial concentrations of both UDCA and chenodeoxycholic acid significantly increased (P < .01), but no significant changes in serum lithocholic acid concentrations were observed.
The authors conclude these data do not support recent suggestions that enhanced biotransformation of UDCA to the hepatotoxic secondary bile acid lithocholic occurs when patients with CFLD are treated with relatively high doses of UDCA.
~ The possibility of UDCA toxicity had been raised (Ooi CY et al 2012) following the recommendation in the European Guidelines for starting UDCA as soon as the diagnosis CFRLD is made (Debray D et al, 2012). The details of the latter are concerns and Carla Colombo’s reply is detailed in the “Topics-Liver” section of this history (Colombo C et al, 2012). In summary of this reply Carla stated“we deem there are no compelling arguments for precluding CF patients to start UDCA early, before the liver disease has potentially progressed to a stage at which changing the course by any medical treatment in no longer possible”. (Please see “Topics – Liver” for the other relevant abstracts reviewed above). Carla Colombo was the first to introduce UDCA for the treatment of CF liver disease in 1990 – before this there was no treatment.
2016 Sivam S; Al-Hindawi Y; Di Michiel J; Moriarty C; Spratt P; Jansz P; Malouf M; Plit M; Pleass H; Havryk A; Bowen D; Haber P; Glanville AR; Bye PT. Liver and lung transplantation in cystic fibrosis: an adult cystic fibrosis centre’s experience. Intern Med J 46(7):852-4, 2016 Jul.[Pubmed] A retrospective analysis was performed on nine patients. Three patients required lung transplantation approximately a decade after liver transplant, and another underwent combined liver and lung transplants (the only fatality). Four additional patients with liver transplants are awaiting assessment for lung transplants. One patient is awaiting combined liver and lung transplants. With increased survival in CF, several patients may require more than single organ transplantation.
2017 Woodruff SA, Sontag MK, Accurso FJ, Sokol RJ, Narkewicz MR. Prevalence of elevated liver enzymes in children with cystic fibrosis diagnosed by newborn screen. J Cyst Fibros. 2017 Jan;16(1):139-145. doi:10.1016/j.jcf.2016.08.002. Epub 2016 Aug 20. [Pubmed]

Samantha Woodruff
Prevalence and risks for elevated liver enzymes have not been studied systematically in children with CF identified by newborn screen. 298 CF children were identified by newborn screen since 1982. AST, ALT and GGT was tested at annual visits. The percent of children with 1 or ≥2 values of elevated AST, ALT and GGT was determined. Relationship of liver enzymes to clinical factors or subsequent liver disease was analysed.
At least one abnormal value for AST (63%), ALT (93%) and ALT ≥1.5× ULN (52%) occurred by 21years of age. Liver enzyme elevations were not correlated with CFTR mutation, meconium ileus or ethnicity. AST and GGT ≥1.5× ULN were associated with later advanced liver disease HR (CI) 6.53 (2.02-21.1) and 4.03 (1.15-13.45), respectively.
– The authors concluded that elevated liver enzymes are common during childhood in CF patients identified by newborn screening. They suggested that elevated AST and GGT may be markers for risk of advanced liver disease. The extent to which URSO was used in these patients was not known. Presumably the use of URSO would be indicated at an early stage in those with persistent elevation of these enzymes – most clinicians would agree that URSO is less effective as liver disease progresses. The authors’ protocol is to prescribe ursodeoxycholic acid after at least 2 elevations of AST, ALT or GGT N 1.5× ULN for more than 6 months. Thus while the continued persistence of elevated liver enzymes beyond 6 months may have been influenced by ursodeoxycholic acid treatment (from 2005), this would have not have had an effect on our definition of elevated enzyme prevalence.
Dr. Samantha Woodruff (figure) is a pediatric gastroenterologist in Aurora, Colorado and is affiliated with multiple hospitals in the area, including Children’s Hospital Colorado and Memorial Hospital.
Ayoub F, Trillo-Alvarez C, Morelli G, Lascano J.Risk factors for hepatic steatosis in adults with cystic fibrosis: Similarities to non-alcoholic fatty liver disease.World J Hepatol. 2018 Jan 27;10(1):34-40. doi: 10.4254/wjh.v10.i1.34. Free PMC Article [Pubmed] To investigate the clinical, biochemical and imaging characteristics of adult cystic fibrosis (CF) patients with hepatic steatosis as compared to normal CF controls. Data was collected on 114 patients meeting inclusion criteria. Seventeen patients (14.9%) were found to have hepatic steatosis on imaging. Being overweight (BMI > 25) (P = 0.019) and having a higher ppFEV1 (75 vs 53, P = 0.037) were significantly associated with hepatic steatosis. Patients with hepatic steatosis had a significantly higher median alanine aminotransferase level (27 vs 19, P = 0.048). None of the hepatic steatosis patients had frank CF liver disease, cirrhosis or portal hypertension. We found no significant association with pancreatic insufficiency or CF related diabetes.
The authors concluded hepatic steatosis appears to be a clinically and phenotypically distinct entity from CF liver disease. The lack of association with malnourishment and the significant association with higher BMI and higher ppFEV1 demonstrate similarities with non-alcoholic fatty liver disease. They suggest ong-term prospective studies are needed to ascertain whether CF hepatic steatosis progresses to fibrosis and cirrhosis.
Dr Fares Walid Ayoub is a physician in the Department of Medicine, University of Florida, Gainsville, Florida
Fawcett LK, Widger J, Henry GM, Ooi CY. Case report: Cholecystoduodenostomy for cholestatic liver disease in a premature infant with cystic fibrosis and short gut syndrome. BMC Pediatr. 2019 Mar 11;19(1):78. doi: 10.1186/s12887-019-1443-5.[Pubmed] Free PMC Article
Cholecystoduodenostomy is a surgical procedure that bypasses the extrahepatic biliary tree and connects the gallbladder directly to the duodenum. This case describes the successful use of this procedure in a novel situation.
A premature (34 weeks gestation) female infant with cystic fibrosis required a laparotomy on day 1 of life due to an intrauterine small bowel perforation. Resection of small bowel and ileostomy formation resulted in short gut syndrome, with 82 cm residual small bowel and intact ileocaecal valve. Post-ileostomy reversal at 2 months old, she developed conjugated hyperbilirubinaemia. Despite conservative management including increased enteral feeding, ursodeoxycholic acid, cholecystostomy drain insertion and flushes, her cholestatic jaundice persisted. A liver biopsy revealed an “obstructive/cholestatic” picture with fibrosis. To avoid further shortening her gut with an hepatoportoenterostomy, cholecystoduodenostomy was performed at 3 months of age with subsequent post-operative improvement and eventual normalisation of her clinical jaundice and liver biochemistry.
This is the first reported case of a cholecystoduodenostomy being used successfully to treat an infant with persistent conjugated hyperbilirubinemia, cystic fibrosis and short gut syndrome. Cholecystoduodenostomy is a treatment option that with further study, may be considered for obstruction of the common bile duct in patients with short gut and/or where a shorter operating time with minimal intervention is preferred.
Cipolli M, Fethney J, Waters D, Zanolla L, Meneghelli I, Dutt S, Assael BM, Gaskin KJ.Occurrence, outcomes and predictors of portal hypertension in cystic fibrosis: A longitudinal prospective birth cohort study. J Cyst Fibros. 2020 May;19(3):455-459. doi: 10.1016/j.jcf.2019.09.016. Epub 2019 Oct 31. [Pubmed]

Kevin Gaskin

Marco Cipolli
Background: The reported prevalence of portal hypertension (PH) in Cystic Fibrosis is variable, incidence rates rarely provided and the utility of liver function tests (LFT’s) early in life to predict PH is questionable. The aims were to (1) determine PH prevalence (P) and incidence rate (IR) and combined mortality transplant (MTX) data in PH vs non-PH patients and (2) to assess association of LFTs in early life with liver disease and PH.
Method: (1) A double centre longitudinal cohort study of 577 CF patients diagnosed by newborn screening (NBS) with annual examinations for PH up to 18.5 years of age (max) was performed over 28 years for P, IR, and MTX data; (2) Cox proportional hazard models were used to assess the association of elevated LFTs on 2 or more occasions over 0-6.5 years and PH.
Results: 51/577(8.8%) developed PH with an average IR of near 3/1000 patient years per 5 year interval representing young, mid and late childhood respectively in patients 3-18 years of age. Combined mortality/liver transplant occurred in 12/51 (23.5%) PH and 25/526 (4.8%) non-PH (p < 0.001). Elevated enzymes particularly GGT (HR:5.71, 95% CI 3.11-10.47); ALT/GGT (HR: 5.56, 95% CI 2.82-10.98); and ALP/GGT (HR: 5.74, 95% CI 2.78-11.86) were associated with the onset of PH.
The authors concluded: This birth cohort with annual examination for PH provides an accurate assessment of the prevalence, and IR of PH and MTX of PH vs non-PH. Early elevated LFTs are associated with onset of MBC/PH
Kevin J Gaskin (2.7.48 – 29.1.21) was Professor at the Department of Gastroenterology/James Fairfax Institute of Paediatric Nutrition, The Children’s Hospital at Westmead, Sydney, Australia
Marco Cipolli is Director of the Cystic Fibrosis Center, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
– A study from the Verona CF Centre in Italy and the Children’s Hospital at Westmead CF clinic Australia – the former introduced neonatal CF screening in the mid-Seventies (Gianni Mastella) and the latter from the early Eighties (Bridget Wilcken). This is the first time a CF neonatal cohort has been followed for 18.5 years assessing the occurrence and subsequent evolution of severe liver disease
Diego A Calvopina , Charlton Noble, Anna Weis, Gunter F Hartel, Louise E Ramm, Fariha Balouch, Manuel A Fernandez-Rojo, Miranda A Coleman, Peter J Lewindon, Grant A Ramm. Supersonic shear-wave elastography and APRI for the detection and staging of liver disease in pediatric cystic fibrosis. J Cyst Fibros. 2020 May;19(3):449-454.doi: 10.1016/j.jcf.2019.06.017. Epub 2019 Jul 11 [Pubmed]

Grant A Ramm
Background: Current diagnostic methods for the diagnosis of Cystic fibrosis (CF)-associated liver disease (CFLD) are non-specific and assessment of disease progression is difficult prior to the advent of advanced disease with portal hypertension. This study investigated the potential of Supersonic shear-wave elastography (SSWE) to non-invasively detect CFLD and assess hepatic fibrosis severity in children with CF.
Methods: 125 children were enrolled in this study including CFLD (n = 55), CF patients with no evidence of liver disease (CFnoLD = 41) and controls (n = 29). CFLD was diagnosed using clinical, biochemical and imaging best-practice guidelines. Advanced CFLD was established by the presence of portal hypertension and/or macronodular cirrhosis on ultrasound. Liver stiffness measurements (LSM) were acquired using SSWE and diagnostic performance for CFLD detection was evaluated alone or combined with aspartate aminotransferase-to-platelet ratio index (APRI).
Results: Liver stiffness measurements were significantly higher in CFLD (8.1 kPa, IQR = 6.7-11.9) versus CFnoLD (6.2 kPa, IQR = 5.6-7.0; P < 0.0001) and Controls (5.3 kPa, IQR = 4.9-5.8; P < 0.0001). LSM was also increased in CFnoLD versus Controls (P = 0.0192). Receiver Operating Characteristic (ROC) curve analysis demonstrated good diagnostic accuracy for LSM in detecting CFLD using a cut-off = 6.85 kPa with an AUC = 0.79 (Sensitivity = 75%, Specificity = 71%, P < 0.0001). APRI also discriminated CFLD (AUC = 0.74, P = 0.004). Classification and regression tree modelling combining LSM + APRI showed 14.8 times greater odds of accurately predicting CFLD (AUC = 0.84). The diagnostic accuracy of SSWE for discriminating advanced disease was excellent with a cut-off = 9.05 kPa (AUC = 0.95; P < 0.0001
Conclusions: SSWE-determined LSM shows good diagnostic accuracy in detecting CFLD in children, which was improved when combined with APRI. SSWE alone discriminates advanced CFLD.
Dr Diego A Calvopina is in the Hepatic Fibrosis Group, QIMR Berghofer Medical Research Institute, Herston Australia and a member of the Faculty of Medicine, The University of Queensland, Brisbane
Professor Grant A Ramm, the corresponding author, is Head of the Hepatic Fibrosis Group d Coordinator of the Cell and Molecular Biology Department at QIMR Berghofer.
Christine Højte , Marianne Hørby Jørgensen, Flemming Jensen, Terese L Katzenstein, Marianne Skov. Extended Screening for Cystic Fibrosis Related Liver Disease Including Elastography Inchildren and Adolescents. Pediatr Gastroenterol Nutr 2020 Jul 28. J doi: 10.1097/MPG.0000000000002872.Online ahead of print. [Pubmed]
Objectives: Advances in treatment of cystic fibrosis (CF) have increased survival and thereby prevalence of patients with liver disease, making chronic liver disease one of the major complications of CF. We describe the prevalence of liver fibrosis, portal hypertension, and liver decompensation by extended screening for cystic fibrosis related liver disease (CFLD) including ultrasound, elastography, and an extended panel of biochemical markers.
Methods: A cross sectional study of CFLD in all pediatric CF patients (1-18 years) from the Copenhagen CF Center. Screening for liver disease included physical examination, biochemical analysis, Vibration-Controlled Transient Elastography (FibroScan), conventional ultrasound, and Real-Time Shear Wave elastography (SWE). Patients were scored according to Williams’ ultrasound scoring scale (WUSS) within six months.
Results: A total of 84 consecutive patients (male sex 46.4%, median age 10.4 years) were included. Eight patients (9.5%) had both ≥ two abnormal results of sonographic methods and ≥ two abnormal biochemical results and were in this study categorized as having manifest CFLD. Manifest CFLD patients were significantly older and had a higher mean value of APRI, but no differences in gender, z-height, z-weight, z-BMI, FEV1% or mean value of bilirubin or albumin were found.
Conclusions: In total eight patients (9.5%) in this pediatric CF population were categorized as having CFLD according to both biochemical and sonographic tests. Consistency was found among the results of FibroScan and SWE. We suggest WUSS and either FibroScan or SWE, combined with GGT as diagnostic markers for CFLD.
From the Copenhagen Cystic Fibrosis Center, Rigshospitalet.
Singh H, Coffey MJ, Ooi CY. Cystic Fibrosis-related Liver Disease is Associated With Increased Disease Burden and Endocrine Comorbidities. J Pediatr Gastroenterol Nutr. 2020 Mar 5. doi: 10.1097/MPG.0000000000002694. [Epub ahead of print] [Pubmed]
Cystic fibrosis-related liver disease (CFLD) is the leading non-pulmonary cause of mortality in cystic fibrosis (CF). The authors evaluated and compared the burden of disease and non-respiratory comorbidities of those with severe CFLD and those without (no CFLD). A retrospective nationwide (Australia) longitudinal review (from 1998 to 2016) of severe CFLD patients compared with no CFLD controls (matched 1 : 1 for age, genotype, pancreatic insufficiency, and center).
One hundred sixty-six patients with severe CFLD and 166 with no CFLD were identified. Forced expiratory volume in 1 second percentage of predicted (FEV1%) was significantly lower in CFLD than no CFLD across all ages. Median (IQR) hospitalizations per patient per year were higher in CFLD than noCFLD for: respiratory indications; gastrointestinal indications); and other indications. In the CFLD cohort, there was increased use of nasogastric and gastrostomy nutritional supplementation Additionally, the CFLD cohort had a higher frequency of bone diseases, osteopenia and osteoporosis, as well as CF-related diabetes.
The authors demonstrated patients with severe CFLD have greater disease burden, with significantly higher number of hospitalizations (both respiratory and non-respiratory indications), nutritional interventions, and are at higher risk of CF-related bone disease and diabetes.
Dr H Singh is in the Department of Gastroenterology, Sydney Children’s Hospital, Randwick.
Siegel MJ, Freeman AJ, Ye W, Palermo JJ, Molleston JP, Paranjape SM, Stoll J, Leung D, Masand P, Karmazyn B, Harned R, Ling SC, Navarro OM, Karnsakul W, Alazraki A, Schwarzenberg SJ, Seidel FG, Towbin A, Alonso EM, Nicholas JL, Murray KF, Otto RK, Sherker AH, Magee JC, Narkewicz MR; CFLD Network. Heterogeneous Liver on Research Ultrasound Identifies Children with Cystic Fibrosis at High Risk of Advanced Liver Disease: Interim Results of a Prospective Observational Case-Controlled Study. J Pediatr. 2020 Feb 11. pii: S0022-3476(19)31712-3. doi: 10.1016/j.jpeds.2019.12.033. [Epub ahead of print] [Pubmed]

Marilyn Siegel
To assess if a heterogeneous pattern on research liver ultrasound examination can identify children at risk for advanced cystic fibrosis (CF) liver disease. Planned 4-year interim analysis of a 9-year multi-center, case-controlled cohort study (Prospective Study of Ultrasound to Predict Hepatic Cirrhosis in CF). Children with pancreatic insufficient CF aged 3-12 years without known cirrhosis, Burkholderia species infection, or short bowel syndrome underwent a screening research ultrasound examination. Participants with a heterogeneous liver ultrasound pattern were matched (by age, Pseudomonas infection status, and center) 1:2 with participants with a normal pattern. Clinical status and laboratory data were obtained annually and research ultrasound examinations biannually. The primary end point was the development of a nodular research ultrasound pattern, a surrogate for advanced CF liver disease.
The final cohort included 55 participants with a heterogeneous liver ultrasound pattern and 116 participants with a normal liver ultrasound pattern. All participants with at least 1 follow-up research ultrasound were included. There were no differences in age or sex between groups at entry. Alanine aminotransferase (42 ± 22 U/L vs 32 ± 19 U/L; P = .0033), gamma glutamyl transpeptidase (36 ± 34 U/L vs 15 ± 8 U/L; P < .001), and aspartate aminotransferase to platelet ratio index (0.7 ± 0.5 vs 0.4 ± 0.2; P < .0001) were higher in participants with a heterogeneous liver ultrasound pattern compared with participants with a normal liver ultrasound pattern. Participants with a heterogeneous liver ultrasound pattern had a 9.1-fold increased incidence (95% CI, 2.7-30.8; P = .0004) of nodular pattern vs a normal liver ultrasound pattern (23% in heterogeneous liver ultrasound pattern vs 2.6% in normal liver ultrasound pattern).
The authors’ main conclusion was that liver research ultrasound examinations can identify children with CF at increased risk for developing advanced CF liver disease.
Dr Marilyn J Siegel is Professor of Radiology and Pediatric Radiology at the Mallinckrodt Institute of Radiology, Washington University School of Medicine, St Louis, MO.
A Lupi , G Barbiero, M Battistel , A Ferrarese, M Loy, P Feltracco, R Stramare, P Burra, M Senzolo. Transjugular intrahepatic portosystemic shunt in non-cirrhotic portal hypertension related to cystic fibrosis in a lung transplant patient. J Cyst Fibros. 2020 Jul 13;S1569-1993(20)30792-X.doi: 10.1016/j.jcf.2020.06.019. Online ahead of print. [Pubmed
Liver involvement is not uncommon in patients with cystic fibrosis (CF). Even if serious complications as non-cirrhotic portal hypertension, cirrhosis and liver failure rarely occur, they are associated with impaired survival and reduced quality of life. Herein, we have reported the first case of a patient with CF and non-cirrhotic portal hypertension who underwent transjugular intrahepatic portosystemic shunt placement for recurrent variceal bleeding after bilateral lung transplantation, and we have reviewed the available literature pertaining to this field.
Dr A Lupi is at the Institute of Radiology, Department of Medicine, Padua University Hospital, Padua, Italy
Keyan Zarei, Mallory R Stroik, Nick D Gansemer, Andrew L Thurman, Lynda S Ostedgaard, Sarah E Ernst, Ian M Thornell, Linda S Powers, Alejandro A Pezzulo, David K Meyerholz, David A Stoltz. Early pathogenesis of cystic fibrosis gallbladder disease in a porcine model. Lab Invest 2020 Nov;100(11):1388-1399. doi: 10.1038/s41374-020-0474-8. Epub 2020 Jul 27 [Pubmed

Keyan Zarei
Hepatobiliary disease causes significant morbidity in people with cystic fibrosis (CF), yet this problem remains understudied. We previously found that newborn CF pigs have microgallbladders with significant luminal obstruction in the absence of infection and consistent inflammation. In this study, we sought to better understand the early pathogenesis of CF pig gallbladder disease. We hypothesized that loss of CFTR would impair gallbladder epithelium anion/liquid secretion and increase mucin production. CFTR was expressed apically in non-CF pig gallbladder epithelium but was absent in CF. CF pig gallbladders lacked cAMP-stimulated anion transport. Using a novel gallbladder epithelial organoid model, we found that Cl– or HCO3– was sufficient for non-CF organoid swelling. This response was absent for non-CF organoids in Cl–/HCO3–-free conditions and in CF. Single-cell RNA-sequencing revealed a single epithelial cell type in non-CF gallbladders that coexpressed CFTR, MUC5AC, and MUC5B. Despite CF gallbladders having increased luminal MUC5AC and MUC5B accumulation, there was no significant difference in the epithelial expression of gel-forming mucins between non-CF and CF pig gallbladders. In conclusion, these data suggest that loss of CFTR-mediated anion transport and fluid secretion contribute to microgallbladder development and luminal mucus accumulation in CF.
Keyan Zarei, a member of David Stolz laboratory in the Department of Internal Medicine and Pappajohn Biomedical Institute, Roy J. and Lucille A. Carver College of Medicine, University of Iowa and the Department of Biomedical Engineering, University of Iowa, Iowa City, IA, 52242, USA.
Katharina Staufer. Current Treatment Options for Cystic Fibrosis-Related Liver Disease. Int J Mol Sci 2020 Nov 14;21(22):8586.doi: 10.3390/ijms21228586. Free PMC article [Pubmed]

Katherina Staufer
Cystic Fibrosis-related liver disease (CFLD) has become a leading cause of morbidity and mortality in patients with Cystic Fibrosis (CF), and affects children and adults. The understanding of the pathogenesis of CFLD is key in order to develop efficacious treatments. However, it remains complex, and has not been clarified to the last. The search for a drug might be additionally complicated due to the diverse clinical picture and lack of a unified definition of CFLD. Although ursodeoxycholic acid has been used for decades, its efficacy in CFLD is controversial, and the potential of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulators and targeted gene therapy in CFLD needs to be defined in the near future. This review focuses on the current knowledge on treatment strategies for CFLD based on pathomechanistic viewpoints.
Dr Katherine Staufer is Head of Transplant Medicine in the Department of Visceral Surgery and Medicine, Inselspital, University Hospital Bern, 3010 Bern, Switzerland;
Carla Colombo, Gianfranco Alicandro, Mark Oliver, Peter J Lewindon, Grant A Ramm, Chee Y Ooi, Federico Alghisi, Nataliya Kashirskaya, Elena Kondratyeva, Fabiola Corti, Rita Padoan, Irina Asherova, Helen Evans, Isabelle de Monestrol, Birgitta Strandvik, Anders Lindblad, CF UDCA study group. Ursodeoxycholic acid and liver disease associated with cystic fibrosis: A multicenter cohort study. J Cyst Fibros 2021 Apr 1;S1569-1993(21)00090-4.doi: 10.1016/j.jcf.2021.03.014.Online ahead of print.[Pubmed]

Carla Colombo
Background: The efficacy and safety of ursodeoxycholic acid (UDCA) for the treatment of liver disease associated with cystic fibrosis (CF) are under discussion, and clinical practice varies among centers. The study aimed at evaluating if the incidence of severe liver disease differs between CF centers routinely prescribing or not prescribing UDCA.
Methods: We carried out a retrospective multicenter cohort study including 1591 CF patients (1192 patients from UDCA-prescribing centers and 399 from non-prescribing centers) born between 1990 and 2007 and followed from birth up to 31 December 2016. We computed the crude cumulative incidence (CCI) of portal hypertension (PH) at the age of 20 years in the two groups and estimated the subdistribution hazard ratio (HR) through a Fine and Gray model.
Results: Over the observation period, 114 patients developed PH: 90 (7.6%) patients followed-up in UDCA prescribing centers and 24 (6.0%) in non-prescribing centers. The CCI of PH at 20 years was 10.1% (95% CI: 7.9-12.3) in UDCA-prescribing and 7.7% (95% CI: 4.6-10.7) in non-prescribing centers. The HR among patients followed in prescribing centers indicated no significant difference in the rate of PH either in the unadjusted model (HR: 1.21, 95% CI: 0.69-2.11) or in the model adjusted for pancreatic insufficiency (HR: 1.28, 95% CI: 0.77-2.12).
Conclusions: CF patients followed-up in UDCA prescribing centers did not show a lower incidence of PH as compared to those followed in centers not prescribing UDCA. These results question the utility of UDCA in reducing the occurrence of severe liver disease in CF.
Professor Carla Colombo is at IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan,
– This is surprising and disappointing. Suggest you read this Liver section in TOPICS where Carla Colombo’s earlier work in 1990 and subsequently is described.
Dr Vito Terlizzi is at the Cystic Fibrosis Regional Reference Center, Department of Paediatric Medicine, Anna Meyer Children’s University, Florence, Italy.
Christina Thornton, Ranjani Somayaji, Michael Parkins, Mark G Swain, Kathleen J Ramos, Christopher H Goss, Abdel A Shaheen. Characteristics and Outcomes of Children With Cystic Fibrosis Hospitalized With Cirrhosis in the United States. Am J Gastroenterol 2021 Apr 29.doi: 10.14309/ajg.0000000000001275. Online ahead of print. [Pubmed]

Christina Thornton
A population-based cohort study of hospitalizations among children with CF using the 2016 Kid’s Inpatient Database. In total, 9,615 admissions were analyzed. Diagnosis of cirrhosis was present in 509 (5.3%) and was significantly associated with increased mortality, length of stay, and hospital charges compared with those without cirrhosis. Hepatic encephalopathy was significantly associated with death in children with cirrhosis.
The authors suggest future interventions should be designed to support children with CF who have cirrhosis to improve clinical outcomes.
Dr Christina Thornton is a Respiratory Fellow at Alberta Health Services
Dr Nagehan Emiralioglu is in the Department of Pediatric Pulmonology, Hacettepe University Faculty of Medicine, Ankara, Turkey
Gunter Flemming, Ulrich Baumann, Richter Nicolas, Vondran Florian, Burkhard Tümmler, Anna-Maria Dittrich, Carsten Müller, Mandy Vogel, Pfister Eva-Doreen. Survival Benefits Following Liver Transplantation – A Matched-Pair Analysis in Pediatric Patients with Cystic Fibrosis. J Pediatr Gastroenterol Nutr 2021 Jun 1.doi: 10.1097/MPG.0000000000003194.Online ahead of print. [Pubmed]
Objectives and study: Cystic fibrosis related liver disease (CFLD) with consecutive cirrhosis is the third most common cause of death in CF patients (3). The aim of this study is to identify the potential long-term benefits of liver transplantation (LTx) in a match-control comparison.
Methods: Retrospective single-center data analysis of all pediatric LTx for CFLD between 1998 and 2014. A control group was selected from the local CF patient registry. Data were collected from case report forms and included clinical and laboratory data, lung function tests, the indication for LTx and details of surgical procedures.
Results: At our institution 23 patients with severe CFLD median age 13.8 years (range 8.7-17.4; 16 boys) underwent LTx between 1998 and 2014. In all patients, normalization of hepatic CF manifestations were achieved after LTx. But obviously there was no significant positive influence on nutritional status. Signs of post-transplant liver steatosis were documented by ultrasound in 17 patients. Liver biopsies after LTx were performed in 19 patients, in 42% (n = 8) of these biopsies a fatty degeneration was observed. 5 patients died after LTx, none because of primary hepatic dysfunction (1 due to post-transplant proliferative disorder, 4 due to infection).Analysis of matched control pairs revealed that liver function, anthropometry, pulmonary function and life expectancy of CFLD patients with LTx are comparable to matched CF peers without CFLD.
Conclusion: Isolated LTx normalizes the hepatic manifestation of CF disease. LTx enables children and adolescents with severe CFLD to have a comparable prognosis in terms of growth, life expectancy and lung function as CF patients without advanced liver involvement. Our data clarifies the long-term perspectives of affected patients.
From the Pediatric Gastroenterology and Hepatology, Hannover Medical School, Hannover, Germany Hospital for Children and Adolescents, University of Leipzig, Germany Department of General, Visceral and Transplant Surgery, Hannover Medical School, Hannover, Germany Pediatric Pneumology, Allergology, and Neonatology, Hannover Medical School, Hannover, Germany
Wolfgang Bernhard, Anna Shunova, Jürgen Machann, Mona Grimmel, Tobias B Haack, Philipp Utz, Ute Graepler-Mainka. Resolution of severe hepatosteatosis in a cystic fibrosis patient with multifactorial choline deficiency: A case report. Nutrition. 2021 May 24;89:111348. doi: 10.1016/j.nut.2021.111348.Online ahead of print. [Pubmed

Wolfgang Bernhard
In cystic fibrosis (CF), 85% to 90% of patients develop exocrine pancreatic insufficiency. De le phosphatidylcholine and choline deficiency. We report on a female patient who has C F and progressive hepatosteatosis from 4.5 y onward. At 22.3 y, the liver comprised 27% fat (2385 mL volume) and transaminases were strongly increased. Plasma choline was 1.9 µmol/L (normal: 8-12 mol/L). Supplementation with 3 × 1g/d choline chloride decreased liver fat and volume (3 mo: 8.2%; 1912 mL) and normalized transaminases. Plasma choline increased to only 5.6 µmol/L upon supplementation, with high trimethylamine oxide levels (12-35 µmol/L; normal: 3 ± 1 mol/L) proving intestinal microbial choline degradation. The patient was homozygous for rs12325817, a frequent single-nucleotide polymorphism in the PEMT gene, associated with severe hepatosteatosis in response to choline deficiency. Resolution of steatosis required 2 y (4.5% fat). Discontinuation/resumption of choline supplementation resulted in rapid relapse/resolution of steatosis, increased transaminases, and abdominal pain.
Prof. Wolfgang Bernhard is in the Department of Neonatology, Children’s Hospital, Eberhard-Karls-University, Tübingen, Germany.
Keyan Zarei, David K Meyerholz, David A StoltzEarly intrahepatic duct defects in a cystic fibrosis porcine model. Physiol Rep. 2021 Jul;9(14):e14978.doi: 10.14814/phy2.14978.[Pubmed]

Keyan Zarei
Hepatobiliary disease causes significant morbidity and mortality in people with cystic fibrosis (CF), yet this problem remains understudied. Previous studies in the newborn CF pig demonstrated decreased bile flow into the small intestine and a microgallbladder with increased luminal mucus and fluid secretion defects. In this study, we examined the intrahepatic bile ducts of the newborn CF pig. We assessed whether our findings from the gallbladder are present elsewhere in the porcine biliary tract and if CF pig cholangiocytes have fluid secretion defects. Immunohistochemistry demonstrated apical CFTR expression in non-CF pig intrahepatic bile ducts of a variety of sizes; CF pig intrahepatic bile ducts lacked CFTR expression. Assessment of serum markers did not reveal significant signs of hepatobiliary disease except for an elevation in direct bilirubin. Quantitative histology demonstrated that CF pigs had smaller bile ducts that more frequently contained luminal mucus. CF intrahepatic cholangiocyte organoids were smaller and lacked cAMP-mediated fluid secretion. Together these data suggest that cholangiocyte fluid secretion is decreased in the CF pig, contributing to structural changes in bile ducts and decreased biliary flow.
Keyan Zarei is a graduate student in the Stolz lab in Department of Internal Medicine, Roy J. and Lucille A. Carver College of Medicine and the Department of Biomedical Engineering, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City, IA, USA.
David Drummond, Jérémy Dana, Laureline Berteloot, Elena K Schneider-Futschik, Frédérique Chedevergne, Céline Bailly-Botuha, Thao Nguyen-Khoa, Mathieu Cornet, Muriel Le Bourgeois, Dominique Debray, Muriel Girard, Isabelle Sermet-Gaudelus. Lumacaftor-ivacaftor effects on cystic fibrosis-related liver involvement in adolescents with homozygous F508 del-CFTR. J Cyst Fibros. 2021 Aug 25;S1569-1993(21)01337-0.doi: 10.1016/j.jcf.2021.07.018. Online ahead of print. [Pubmed]

David Drummond
Background: The effects of lumacaftor-ivacaftor on cystic fibrosis transmembrane conductance regulator (CFTR)-associated liver disease remain unclear. The objective of the study was to describe the effect of this treatment on features of liver involvement in a cystic fibrosis (CF) adolescent population homozygous for F508del.
Methods: Clinical characteristics, liver blood tests, abdominal ultrasonography (US), and pancreas and liver proton density fat fraction (PDFF) by magnetic resonance imaging, were obtained at treatment initiation and at 12 months for all patients. Biomarkers of CFTR activity (sweat chloride test, nasal potential difference, and intestinal current measurement) were assessed at initiation and at 6 months therapy.
Results: Of the 37 patients who started ivacaftor/lumacaftor treatment, 28 were eligible for analysis. In this group, before treatment initiation, 4 patients were diagnosed with multinodular liver and portal hypertension, 19 with other forms of CF liver involvement, and 5 with no signs of liver involvement. During treatment, no hepatic adverse reactions were documented, and no patient developed liver failure. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gammaglutamyl transferase (GGT) decreased significantly following initiation of lumacaftor-ivacaftor, and remained so after 12 months treatment. This was not correlated with changes in clinical status, liver and pancreas US and PDFF, fecal elastase, or lumacaftor-ivacaftor serum levels. The most “responsive” patients demonstrated a significant increase in biomarkers of CFTR activity.
Conclusions: These results may suggest a potential beneficial effect of CFTR modulators on CF liver disease and warrant further investigation in larger, prospective studies.
Dr David Drummond is a paediatrician at Service de Pneumologie et Allergologie Pédiatriques, Centre de Référence Maladies Rares Mucoviscidose et Maladies apparentées, Centre de Ressources et de Compétences pour la Mucoviscidose, Hôpital Necker Enfants Malades, Assistance Publique-Hôpitaux de Paris (AP-HP)-Centre, Université de Paris, Paris, France; Université de Paris, Paris, France.
Yasmeen Obeidat, Davinder Singh, Saba AlTarawneh, Joseph Simmons, Adnan Elghezewi, Eva Patton-Tackett, Wesam Frandah.Ascending Cholangitis Caused by Methicillin-Resistant Staphylococcus aureus Species in a Patient With Cystic Fibrosis. Cureus 2021 Aug 10;13(8):e17045doi: 10.7759/cureus.17045.eCollection 2021 Aug. Free PMC article [Pubmed]

Yasmeen Obeidat
Ascending cholangitis is a bacterial infection of the extra-hepatic biliary system and presents as a life-threatening systemic condition. Increased bacterial loads and biliary obstruction favor bacterial translocation into the vascular and lymphatic systems. Common organisms isolated are Escherichia Coli, Klebsiella, Enterococcus species, and Enterobacter species. Methicillin-resistant Staphylococcus aureus (MRSA) is a rare isolate in ascending cholangitis. We present a case of a 24-year-old patient with cystic fibrosis who presented with epigastric abdominal pain, low-grade fever, jaundice, dark urine, and nausea for two days. Initial workup revealed elevated liver enzymes, hyperbilirubinemia, leukocytosis, and an ultrasound which showed common bile duct dilation to 14 mm with choledocholithiasis. He underwent endoscopic retrograde cholangiopancreatography (ERCP) with stone extraction and bile fluid culture. Cultures grew out MRSA and the patient was treated with appropriate antibiotic therapy. The mainstay of therapy for ascending cholangitis is adequate hydration, antibiotics, and biliary decompression. Early recognition of the offending organism is critical in guiding therapy. Current guidelines focus on the empiric treatment of Gram-negative and anaerobic bacteria. Clinicians should be aware of the possibility of less common pathogens (such as MRSA), especially in a patient who is decompensating despite antibiotic therapy.
Dr Yasmeen Obeidat is a Resident in Internal medicine in the department Internal Medicine, Marshall University Joan C. Edwards School of Medicine, Huntington, USA.
Sibel Yavuz, Ferhat C Pişkin, Cemil Oktay, Gökhan Tümgör. of hepatic involvement by two-dimensional shear wave elastography in paediatric patients with cystic fibrosis. J Paediatr Child Health. 2021 Sep 14.doi: 10.1111/jpc.15741.Online ahead of print.[Pubmed]

Sibel Yavuz
Aim:This study aimed to investigate parenchymal changes in the liver in paediatric patients with cystic fibrosis (CF) and to analyse diagnostic performance of two-dimensional shear wave elastography (2D-SWE) for the detection of hepatic involvement.
Methods:Patients with CF treated and followed at our centre were evaluated prospectively. All patients underwent liver tissue stiffness (TS) measurements by 2D-SWE, in addition to routine clinical assessments, laboratory work-up and abdominal ultrasound imaging. Data from patients with CF were compared with healthy control subjects.
Results:This study included 39 patients with CF and 37 healthy controls. Patients had a mean body weight of 29.9 (16.6-55) kg, mean age of 9 (5-17) years, mean height of 130 (107-172) cm and a mean body mass index of 16.1 (12.8-21.4) kg/m2 . Average SWE values of the liver were 1.02 (0.70-1.60) m/s in patients with CF (n = 39) and 0.89 (0.60-1.35) m/s in healthy controls (n = 37). Cystic fibrosis patients had significantly increased tissue stiffness by liver elastography compared to controls (P = 0.005).
Conclusion:Parenchymal liver changes may occur early in cystic fibrosis, which cannot be detected by conventional ultrasonography but may be demonstrated by 2D-SWE. Based on this cross-sectional study, 2D-SWE may be a promising, simple and non-invasive modality for objective monitoring of patients with cystic fibrosis who require lifelong follow-up, by providing numerical data for tissue stiffness early in the disease.
Dr Sibel Yavuz is in the Department of Pediatric Gastroenterology, Cukurova University Medical Faculty, Adana, Turkey.
Kevin E Bove, Anas Bernieh, Jennifer Picarsic, Joseph P Cox, Edmund Yang, Philip C Mantor, Ameet Thaker, Lauren Lazar, Meghana Sathe, Stephen Megison. Hypoplasia of Extrahepatic Biliary Tree and Intrahepatic Cholangiolopathy in Cystic Fibrosis Imperfectly Mimic Biliary Atresia in 4 Infants With Cystic Fibrosis and Kasai Portoenterostomy. Am J Surg Pathol. 2021 Sep 13 doi: 10.1097/PAS.0000000000001803. Online ahead of print. [Pubmed]
Four male infants with cystic fibrosis and prolonged neonatal jaundice underwent Kasai procedure to relieve biliary obstruction due to apparent biliary atresia. The excised remnants had viscid mucus accumulation in hypoplastic gallbladders and distended peribiliary glands. Main hepatic ducts were narrow and/or malformed. Microscopic differences between the gallbladder and extrahepatic bile ducts in cystic fibrosis and sporadic biliary atresia were unequivocal, despite some histologic overlap; no erosive or fibro-obliterative lesions typical of biliary atresia were seen. Common in liver, biopsies were small duct cholangiopathy with intense focal cholangiolitis and massive accumulation of ceroid pigment within damaged cholangiocytes, and in portal macrophages, portal fibrosis, and unequivocal features of large duct obstruction were inconspicuous compared with biliary atresia. Plugs of bile in small ducts tended to be pale and strongly periodic acid-Schiff-reactive in cystic fibrosis. Distinguishing the liver lesion from that of biliary atresia is challenging but possible. Liver biopsies from 2 additional infants with cystic fibrosis and prolonged jaundice that spontaneously resolved showed a similar small duct cholangiopathy. Small gallbladders and extrahepatic ducts challenge surgical judgment as findings in liver biopsies challenge the pathologist. The decision to perform a Kasai procedure is reasonable when mimicry of biliary atresia is grossly complete. We hypothesize that a disorder of bile volume/flow during development and/or early infancy linked to the CFTR mutation alone or in combination with the stresses of neonatal intensive care causes destructive cholangiolitis and intrahepatic reduction of bile flow with secondary hypoplasia of extrahepatic biliary structures.
Dr Kevin E Bove is a paediatric staff pathologist and professor in the Division of Pathology Department of Pediatric Surgery, Cincinnati Children’s Hospital, Cincinnati,
Varinder S Athwal, Jennifer A Scott, Emer Fitzpatrick, Marion Rowland. Emerging clinical perspectives in cystic fibrosis liver disease. Curr Opin Pulm Med 2021 Sep 1.doi: 10.1097/MCP.0000000000000824. Online ahead of print. [Pubmed]

Varinder Athwal
Purpose of review: Liver disease (CFLD) as a complication of cystic fibrosis is recognized as a more severe disease phenotype in both children and adults. We review recent advances in understanding the disease mechanism and consider the implications of new strategies for the diagnosis and management of cystic fibrosis in those with evidence of clinically significant liver disease.
Recent findings: Evidence suggests that the prevalence of CFLD has not declined with the introduction of newborn screening. Furthermore, children with CFLD, who have been diagnosed with cystic fibrosis following newborn screening continue to have a much higher mortality rate compared with those with no liver disease. There is further data suggesting noncirrhotic obliterative portal venopathy as the predominant pathological mechanism in the majority of children and young adults receiving a liver transplantation. Little progress has been made in developing an accurate noninvasive test for early diagnosis or monitoring disease progression in CFLD. The benefit of new modulator therapies is not well understood in those with established CFLD, whereas the risk of hepatotoxicity as a complication of treatment must be carefully monitored.
Summary: Better understanding of the pathophysiology of CFLD would allow a standardized approach to diagnosis, with the potential to improve outcomes for those with CFLD.
Dr Varinder S Athwal is Senior Clinical Lecturer and Consultat the Gastroenterology and Hepatology, Manchester University NHS Foundation Trust
Jérémy Dana, Dominique Debray ,Aurélie Beaufrère, Sophie Hillaire , Monique Fabre Caroline Reinhold, Thomas Baumert, Laureline Berteloot, Valérie Vilgrain. Cystic fibrosis-related liver disease: clinical presentations, diagnostic and monitoring approaches in the era of CFTR modulator therapies. J Hepatol 2021 Oct 19;S0168-8278(21)02115-2 doi: 10.1016/j.jhep.2021.09.042.Online ahead of print. [Pubmed]
Cystic fibrosis (CF) is the most common autosomal recessive disease in the Caucasian population. Cystic Fibrosis-related Liver Disease (CFLD) is defined by the pathogenesis related to the underlying CFTR defect in biliary epithelial cells. CFLD needs to be distinguished from other liver manifestations that may not have any pathological significance. The clinical and histological presentation as well as the severity of CFLD varies. The main histological presentation of CFLD is focal biliary fibrosis, which is usually asymptomatic. Portal hypertension develops in a minority of cases (about 10%) and may require specific management including liver transplantation for end-stage liver disease. Portal hypertension is usually the result of the progression of focal biliary fibrosis to multilobular cirrhosis during childhood. Nevertheless, non-cirrhotic portal hypertension as a result of porto-sinusoidal vascular disease is now identified increasingly more frequently in these patients, mainly in young adults. To evaluate the effect of new CFTR modulator therapies on the liver, the spectrum of hepatobiliary involvement must first be precisely classified. This paper discusses the phenotypical features of CFLD, its underlying physiopathology and relevant diagnostic and follow-up approaches with a special focus on imaging.
Jérémy Dana is in the Department of Radiology, Beaujon Hospital, Université de Paris, Clichy, France; Université de Strasbourg, Strasbourg, France; Inserm, U1110, Institut de Recherche sur Les Maladies Virales et Hépatiques, Strasbourg, France; IHU-Strasbourg (Institut Hospitalo-Universitaire), Pôle Hépato-digestif, Nouvel Hôpital Civil, Strasbourg, France.
Mahan Salehi , Mubashar Iqbal, Asha Dube, Amer AlJoudeh, Frank Edenborough.Delayed hepatic necrosis in a cystic fibrosis patient taking Elexacaftor/Tezacaftor/Ivacaftor (Kaftrio)Respir Med Case Rep 2021 Nov 10;34:101553.doi: 10.1016/j.rmcr.2021.101553. eCollection 2021 Free PMC article. [Pubmed]

Frank Edenborough

Mahan Salehi
The introduction of Cystic Fibrosis Trans Regulatory modulator (CFTRm) drugs has seen a transformation in Cystic Fibrosis (CF) treatment. This has led to a significant improvement in lung function and quality of life with the potential for a real impact on life expectancy. Transient mild to moderate hepatic transaminitis is a well-known side effect of CFTRm drugs, which often improves on cessation and may not recur following the re-institution of the drug. We describe a case of transaminitis developing nine months after the initiation of Kaftrio, which progressed to liver necrosis despite stopping Kaftrio and took many months to resolve. The patient had experienced significant improvement in lung function and overall health while on Kaftrio and deteriorated when it was stopped. He was keen to restart; however, Kaftrio was not reinstated due to the potential risk of acute liver failure.
Mahan Salehi an Academic Foundation Doctor at the Sheffield Teaching Hospitals, Adult Cystic Fibrosis Centre, Sheffield, UK.
Diego A Calvopina, Peter J Lewindon, Louise E Ramm, Charlton Noble , Gunter F Hartel, Daniel H Leung, Grant A Ramm. Gamma-glutamyl transpeptidase-to-platelet ratio as a biomarker of liver disease and hepatic fibrosis severity in paediatric Cystic Fibrosis. J Cyst Fibros 2021 Dec 22;S1569-1993(21)02171-8. doi: 10.1016/j.jcf.2021.10.014.Online ahead of print.[Pubmed]

Diego Calvopina
Background: Cystic fibrosis (CF)-associated liver disease (CFLD) causes significant morbidity and mortality in children with CF. Diagnosis of liver disease prior to development of cirrhosis or portal hypertension (PHT) is challenging. While imaging modalities using Elastography show great promise they are still not widely available to all clinicians. This study investigated gamma-glutamyl transpeptidase-to-platelet ratio (GPR) as a non-invasive biomarker to detect liver disease and stage fibrosis severity in children with CF.
Results: GPR was significantly increased in CFLD versus CFnoLD (0.33 [0.19-0.96] vs. 0.15 [0.11-0.21], P<0.0001). GPR demonstrated good diagnostic utility for detecting CFLD with an area under the curve (AUC) of 0.81 (95% confidence Interval [CI] [0.75-0.87]; P<0.0001), with sensitivity of 74% and specificity of 73%, using a cut-off of 0.20. GPR increased with increasing hepatic fibrosis stage. GPR discriminated both moderate-advanced (F2-F4) fibrosis vs. F0-F1 (AUC=0.82; 95%CI [0.71-0.94]; P<0.0001) and advanced (F3-F4) fibrosis vs. F0-F2 (AUC=0.77; 95%CI [0.64-0.90]; P = 0.004), with a cut-off 0.32 and 0.61, respectively. An elevated GPR of >0.84 was predictive of PHT at diagnosis of CFLD (AUC=0.81; 95%CI [0.67-0.95]; P = 0.0003).
Conclusions: GPR demonstrates good diagnostic utility for assessing the presence of liver disease, PHT and hepatic fibrosis severity in children with CF. These findings will aid in better identification of patients at risk for CF-related liver involvement and the potential for more targeted and timely follow-up and treatment.
Diego A Calvopina is a research scientist in the Hepatic Fibrosis Group, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, QLD 4006, Australia
Raphael Enaud Eric Frison,, Sophie Missonnier, Aude Fischer, Victor de Ledinghen, Paul Perez, Stéphanie Bui, Michael Fayon, Jean-François Chateil, Thierry Lamireau. Cystic fibrosis and noninvasive liver fibrosis assessment methods in children. Pediatr Res 2022 Jan;91(1):223-229.doi: 10.1038/s41390-021-01427-4. Epub 2021 Mar 17. [Pubmed]

Rapheal Enaud
Background: Noninvasive assessments of liver fibrosis are currently used to evaluate cystic fibrosis (CF)-related liver disease. However, there is scarce data regarding their repeatability and reproducibility, especially in children with CF. The present study aimed to evaluate the repeatability and reproducibility of transient elastography (TE) (FibroScan®) and point shear-wave elastography using virtual touch quantification (pSWE VTQ) in children with CF.
Methods: TE and pSWE VTQ were performed in 56 children with CF by two different operators. Analysis of repeatability and reproducibility was available in 33 patients for TE and 46 patients for pSWE VTQ. Intra- and interobserver agreement were assessed using the intraclass correlation coefficient (ICC) and their 95% confidence interval (CI), and Bland and Altman graphs.
Results: For TE, ICC was 0.91 (0.83-0.95) for intraobserver agreement and 0.92 (95% CI: 0.86-0.96) for interobserver agreement. For pSWE VTQ, ICC was 0.83 (0.72-0.90) for intraobserver agreement and 0.67 (0.48-0.80) for interobserver agreement.
Conclusions: Both technics can be proposed in the follow-up of patients, according to their availability in CF centers.
Impact: This study shows that TE and pSWE VTQ are reliable methods to evaluate liver fibrosis in children with CF. This study shows for the first time that TE and pSWE VTQ are both repeatable and reproducible in children with CF. These data indicate that both TE and pSWE VTQ can be proposed for the follow-up of patients with CF, according to their availability in each CF center.
Dr Raphael Enaud is at the following – Pediatric Hepatology and Gastroenterology Unit, Bordeaux University Hospital, Pellegrin-Enfants Hospital, Bordeaux, France.
Bordeaux University Hospital, Pellegrin-Enfants Hospital, Pediatric Cystic Fibrosis-Center (CRCM), Bordeaux, France.
INSERM, Centre de Recherche Cardio-thoracique de Bordeaux (U1045), University of Bordeaux, Bordeaux, France
Hunter Ragan, Elizabeth Autry, Taryn Bomersback, Jennifer Hewlett, Lauren Kormelink, Julie Safirstein, Laura Shanley, Lisa Lubsch. The use of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis post-liver transplant: A case series. Pediatr Pulmonol 2022 Feb;57(2):411-417. doi: 10.1002/ppul.25779. Epub 2021 Dec 12. [Pubmed]
Introduction: Cystic fibrosis (CF)-related liver disease (CFLD) manifests as a wide spectrum of hepatobiliary disease and can progress to need liver transplantation. Elexacaftor/tezacaftor/ivacaftor (elx/tez/iva) is a cystic fibrosis transmembrane conductance regulator modulator that has superior efficacy compared to previously approved modulators. Use of elx/tez/iva, should be approached with caution in individuals with CFLD or following liver transplantation due to possible increases in liver function tests (LFTs) and drug-drug interactions with several immunosuppressant medications.
Objective: The purpose of this case series is to explore if the use of elx/tez/iva is safe and tolerable in patients with CF postliver transplantation.
Methods: A retrospective case series including patients prescribed elx/tez/iva following liver transplantation and an immunosuppressive regimen consisting of drug therapy metabolized by P-glycoprotein was completed.
Results: Ten patients at six CF centers with a median age of 22.1 years (range 14-43.4 years) and the median time from the transplant of 6.9 years (range 0.6-22 years) were included. Most patients (8, 80%) received a reduced or full dose of elx/tez/iva for a mean duration of 10.4 months (range 7-12 months). Fluctuations in LFTs occurred in all patients (10, 100%) and led to therapy discontinuation in two patients (20%). Elx/tez/iva initiation resulted in elevations in tacrolimus trough concentration in seven patients (70%). Most patients who tolerated elx/tez/iva had symptomatic and quality of life improvement, increased body mass index, and maintained or improved lung function.
Conclusion: Initiation of elx/tez/iva in patients with CF who received liver transplantation may be safe with clinical benefits.
Hunter Ragan is at the Goldfarb School of Nursing at Barnes-Jewish College, St. Louis, Missouri, USA.
Gary J Galante. Defining and Treating Cystic Fibrosis Liver Disease: Some Things Old and Some Things New. J Cyst Fibros 2022 Mar;21(2):199-201. doi: 10.1016/j.jcf.2022.03.004. [Pubmed]

Gary J Galante
This is a review of the present situation regarding liver involvement. The authors conclude “that although we may not have significant advancement in the prevention of CFLD and its progression to clinically significant outcomes, it is undoubtedly an exciting time in therapeutics with the advent and widespread use of CFTR modulators. While hepatotoxicity remains a concern and surveillance on treatment is prudent given the potential for idiosyncratic reactions and drug-drug interactions, significant liver injury is rare and generally resolves with medication discontinuation. Moreover, early and consistent use could theoretically prevent or limit CFLD, supported by some clinical data. As is generally the case for therapeutic studies in CFLD, more prospective well-designed studies are needed to examine clinically significant outcomes over the long-term. However, there is reason for cautious optimism with novel CFTR modulators, on the background of improving survival in CFLD reflective of ongoing improvements in overall CF care”
Gary J Galante is a gastroenterologist and Clinical Assistant Professor in the Department of Pediatrics, Alberta Children’s Hospital, University of Calgary, Calgary, Alberta, Canada.
Mitchell L Ramsey, Michael R Wellner, Kyle Porter, Stephen E Kirkby, Susan S Li , Luis F Lara , Sean G Kelly, A James Hanje, Lindsay A Sobotka. Cystic fibrosis patients on cystic fibrosis transmembrane conductance regulator modulators have a reduced incidence of cirrhosis. World J Hepatol 2022 Feb 27;14(2):411-419.doi: 10.4254/wjh.v14.i2.411. Free PMC article [Pubmed]

Mitchell Ramsey
Background: Cystic fibrosis transmembrane conductance regulator (CFTR) modulators significantly improve pulmonary function in patients with cystic fibrosis (CF) but the effect on hepatobiliary outcomes remains unknown. We hypothesized that CF patients on CFTR modulators would have a decreased incidence of cirrhosis compared to patients not on CFTR modulators or on ursodiol.
Aim: To investigate the effect of CFTR modulators on the development of cirrhosis in patients with CF.
Methods: A retrospective analysis was performed using Truven MarketScan from January 2012 through December 2017 including all patients with a diagnosis of CF. Patients were excluded if they underwent a liver transplantation or if they had other etiologies of liver disease including viral hepatitis or alcohol use. Subjects were grouped by use of CFTR modulators, ursodiol, dual therapy, or no therapy. The primary outcome was development of cirrhosis. Kaplan-Meier curves estimated the incidence of cirrhosis and log-rank tests compared incidence curves between treatment groups.
Results: A total of 7201 patients were included, of which 955 (12.6%) used a CFTR modulator, 529 (7.0%) used ursodiol, 105 (1.4%) used combination therapy, and 5612 (74.3%) used neither therapy. The incidence of cirrhosis was 0.1% at 1 year and 0.7% at 4 years in untreated patients, 5.9% and 10.1% in the Ursodiol group, and 1.0% and 1.0% in patients who received both therapies. No patient treated with CFTR modulators alone developed cirrhosis. Patients on CFTR modulators alone had lower cirrhosis incidence than untreated patients (P = 0.05), patients on Ursodiol (P < 0.001), and patients on dual therapy (P = 0.003). The highest incidence of cirrhosis was found among patients treated with Ursodiol alone, compared to untreated patients (P < 0.001) or patients on Ursodiol and CFTR modulators (P = 0.01).
Conclusion: CFTR modulators are associated with a reduction in the incidence of cirrhosis compared to other therapies in patients with CF.
Dr Mitchell L Ramsey is in the Department of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center, Columbus, OH 43210, United States.
Daniel H Leung , Wen Ye, Sarah J Schwarzenberg, A Jay Freeman , Joseph J Palermo, Alexander Weymann,et al, CFLD Research Network. Long-term follow-up and liver outcomes in children with cystic fibrosis and nodular liver on ultrasound in a multi-center study. J Cyst Fibros 2022 Aug 16;S1569-1993(22)00635-X.doi: 10.1016/j.jcf.2022.07.017.Online ahead of print [Pubmed]

Daniel H Leung
Background: Nodular liver (NOD) in cystic fibrosis (CF) suggests advanced CF liver disease (aCFLD); little is known about progression of liver disease (LD) after detection of sonographic NOD.
Methods: Clinical, laboratory, and ultrasound (US) data from Prediction by Ultrasound of the Risk of Hepatic Cirrhosis in CFLD Study participants with NOD at screening or follow-up were compared with normal (NL). Linear mixed effects models were used for risk factors for LD progression and Kaplan-Meier estimator for time-to-event.
Results: 54 children with NOD (22 screening, 32 follow-up) and 112 NL were evaluated. Baseline (BL) and trajectory of forced expiratory volume, forced vital capacity, height/BMI z-scores were similar in NOD vs NL. Platelets were lower in NOD at BL (250 vs 331×103/microL; p < 0.001) and decreased by 8600/year vs 2500 in NL. Mean AST to Platelet Ratio Index (1.1 vs 0.4; p < 0.001), Fibrosis-4 Index (0.4 vs 0.2, p < 0.001), and spleen size z-score (SSZ) [1.5 vs 0.02; p < 0.001] were higher in NOD at BL; SSZ increased by 0.5 unit/year in NOD vs 0.1 unit/year in NL. Median liver stiffness (LSM) by transient elastography was higher in NOD (8.2 kPa, IQR 6-11.8) vs NL (5.3, 4.2-7, p < 0.0001). Over 6.3 years follow-up (1.3-10.3), 6 NOD had esophageal varices (cumulative incidence in 10 years: 20%; 95% CI: 0.0%, 40.0%), 2 had variceal bleeding, and 2 underwent liver transplantation; none had ascites or hepatic encephalopathy. No NL experienced liver-related events.
Conclusions: Nodular liver (NOD) developed clinically evident portal hypertension faster than NL without worse growth or lung disease.
Dr Daniel H Leung is Associate Professor in the Department of Pediatrics, Baylor College of Medicine, Division of Gastroenterology, Hepatology and Nutrition, Texas Children’s Hospital, 6621 Fannin St, CCC 1010.00, Houston, TX 77030, USA.
Harold Tabori , Jochen Schneider , Stefan Lüth , Carlos Zagoya , Anton Barucha , Thomas Lehmann , Eberhard Kauf , Astrid Barth , Jochen G Mainz Elevated Levels of Toxic Bile Acids in Serum of Cystic Fibrosis Patients with CFTR Mutations Causing Pancreatic Insufficiency. Int J Mol Sci
. 2022 Oct 18;23(20):12436. doi: 10.3390/ijms232012436. pubmed.ncbi.nlm.nih.gov/36293293/

Harold Tabori researchgate.net
Hepatobiliary involvement is a hallmark in cystic fibrosis (CF), as the causative CF Transmembrane Conductance Regulator (CFTR) defect is expressed in the biliary tree. However, bile acid (BA) compositions in regard to pancreatic insufficiency, which is present at an early stage in about 85% of CF patients, have not been satisfactorily understood. We assess the pattern of serum BAs in people with CF (pwCF) without CFTR modulator therapy in regard to pancreatic insufficiency and the CFTR genotype. In 47 pwCF, 10 free and 12 taurine- and glycine-conjugated BAs in serum were prospectively assessed. Findings were related to genotype, pancreatic insufficiency prevalence (PIP)-score, and hepatic involvement indicated by serum liver enzymes, as well as clinical and ultrasound criteria for CF-related liver disease. Serum concentrations of total primary BAs and free cholic acid (CA) were significantly higher in pwCF with higher PIP-scores (p = 0.025, p = 0.009, respectively). Higher total BAs were seen in pwCF with PIP-scores ≥0.88 (p = 0.033) and with pancreatic insufficiency (p = 0.034). Free CA was higher in patients with CF-related liver involvement without cirrhosis, compared to pwCF without liver disease (2.3-fold, p = 0.036). pwCF with severe CFTR genotypes, as assessed by the PIP-score, reveals more toxic BA compositions in serum. Subsequent studies assessing changes in BA homeostasis during new highly effective CFTR-modulating therapies are of high interest.
Dr Harold Tabori is at the Cystic Fibrosis Center, Brandenburg Medical School (MHB) University, Klinikum Westbrandenburg, 14770 Brandenburg an der Havel, Germany.
and Internal Medicine, Alexianer Hedwigshöhe Hospital, 12526 Berlin, Germany .
Almudena Marinero Martínez-Lázaro, Rosa María Girón Moreno, Fernando Casals Seoane, Óscar Cano-Valderrama, Luisa García-Buey. Cystic fibrosis with liver involvement in adults has a benign course. Results from a tertiary referral centre cohort. Rev Esp Enferm Dig. 2022 Nov 10;114. doi: 10.17235/reed.2022.9289/2022. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36353964/
Background: Cystic Fibrosis Liver Disease is a poorly understood entity, especially in adults, in terms of its real prevalence, natural history and diagnostic criteria, despite being the most important extrapulmonary cause of mortality. The aim was to evaluate the prevalence, characteristics and potential risk factors of liver disease in adults with cystic fibrosis, according to two diagnostic criteria accepted in the scientific literature.
Methods: Patients were recruited in a tertiary referral hospital, and laboratory, ultrasound, non-invasive liver fibrosis tests (AST to Platelet Ratio Index; Fibrosis-4 Index) and transient elastography (Fibroscan) were performed. The proportion of patients with liver disease according to the Debray and Koh criteria were evaluated.
Results: 95 patients were included, 48 (50.5%) females, with a mean age of 30.4 (28.6-32.2) years. According to the Debray criteria, 6 (6.3%) patients presented liver disease. According to the Koh criteria, prevalence increased up to 8.4%, being statistically different from the 25% value described in other published series (p = 0.005). Seven (7.5%) presented ultrasonographic chronic liver disease. Eleven (13%) presented liver fibrosis according to the APRI score; 95 (100%) had a normal FIB-4 value. Mean liver stiffness value was 4.4 (4.1-4.7) kPa. FEV1 (OR=0.16, p 0.05), meconium ileus (OR=14.16, p 0.002), platelets (Pearson coefficient -0.25, p 0.05) and younger age (Pearson coefficient -0.19, p 0.05) were risk factors.
Conclusions: Prevalence and severity of liver disease in adult cystic fibrosis patients were lower than expected. Meconium ileus, platelets, age and respiratory function were confirmed as risk factors associated to cystic fibrosis liver disease.
Almudena Marinero Martínez-Lázaro is at Digestivo, Hospital Universitario Clínico San Carlos .
Steven Levitte, Yonathan Fuchs, Russell Wise , Zachary M Sellers. Effects of CFTR modulators on serum biomarkers of liver fibrosis in children with cystic fibrosis. J Hepatol Commun. 2023 Jan 20;7(2):e0010. doi: 10.1097/HC9.0000000000000010. Free article pubmed.ncbi.nlm.nih.gov/36662672/

Steven Levitte. LinkedIn
The cystic fibrosis (CF) transmembrane conductance regulator corrector/potentiator combinations lumacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor improve sweat chloride, pulmonary function, and nutrition. Yet it is unclear whether they may also impact the progression of liver fibrosis, which is a substantial source of morbidity and mortality for patients with CF. We conducted a retrospective, single-center analysis of children and adolescents with CF treated with lumacaftor/ivacaftor and/or elexacaftor/tezacaftor/ivacaftor therapy, focusing on alterations in liver function tests and fibrosis indices using previously-established thresholds that corresponded with increased liver elastography.
In pairwise comparisons of before and during treatment timepoints, we found that those with CF-associated liver involvement experienced significant decreases in gamma-glutamyl transferase, aspartate aminotransferase-to-platelet index, and gamma-glutamyl transferase-to-platelet ratio while on lumacaftor/ivacaftor. These differences were not observed in patients treated with elexacaftor/tezacaftor/ivacaftor, nor were they observed in patients without underlying CF-associated liver disease. These results provide the first evidence that lumacaftor/ivacaftor may improve liver fibrosis in children and adolescents with CF and suggest it may be beneficial in the treatment of CF-associated liver disease
Steven Levitt is in the Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Stanford Univ
Qingtian Wu, Xiubin Liang Xia Hou, Zhenfeng Song, Mohamad Bouhamdan, Yining Qiu , Yui Koike , Carthic Rajagopalan, Hong-Guang Wei, Hong Jiang, Gerry Hish, Jifeng Zhang, Y Eugene Chen, Jian-Ping Jin, Jie Xu , Kezhong Zhang, Fei Sun. Cystic fibrosis rabbits develop spontaneous hepatobiliary lesions and CF-associated liver disease (CFLD)-like phenotypes. PNAS Nexus. 2022 Dec 23;2(1):pgac306. doi: 10.1093/pnasnexus/pgac306. eCollection 2023 Jan. 36712930 Free PMC article pubmed.ncbi.nlm.nih.gov/36712930/
Erratum in Correction to: Volume 2 Issue 1 of PNAS Nexus. PNAS Nexus. 2023 Jan 27;2(1):pgad016. doi: 10.1093/pnasnexus/pgad016. eCollection 2023 Jan. pubmed.ncbi.nlm.nih.gov/36744020/
Cystic fibrosis (CF) is an autosomal recessive genetic disease affecting multiple organs. Approximately 30% CF patients develop CF-related liver disease (CFLD), which is the third most common cause of morbidity and mortality of CF. CFLD is progressive, and many of the severe forms eventually need liver transplantation. The mechanistic studies and therapeutic interventions to CFLD are unfortunately very limited. Utilizing the CRISPR/Cas9 technology, we recently generated CF rabbits by introducing mutations to the rabbit CF transmembrane conductance regulator (CFTR) gene. Here we report the liver phenotypes and mechanistic insights into the liver pathogenesis in these animals. CF rabbits develop spontaneous hepatobiliary lesions and abnormal biliary secretion accompanied with altered bile acid profiles. They exhibit nonalcoholic steatohepatitis (NASH)-like phenotypes, characterized by hepatic inflammation, steatosis, and fibrosis, as well as altered lipid profiles and diminished glycogen storage. Mechanistically, our data reveal that multiple stress-induced metabolic regulators involved in hepatic lipid homeostasis were up-regulated in the livers of CF-rabbits, and that endoplasmic reticulum (ER) stress response mediated through IRE1α-XBP1 axis as well as NF-κB- and JNK-mediated inflammatory responses prevail in CF rabbit livers.
These findings show that CF rabbits manifest many CFLD-like phenotypes and suggest targeting hepatic ER stress and inflammatory pathways for potential CFLD treatment
Qingtian Wu is in the Department of Physiology, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Daniel H Tewkesbury, Varinder Athwal, Rowland J Bright-Thomas, Andrew M Jones, Peter J Barry Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on liver tests at a large single adult cystic fibrosis centre. J Cyst Fibros. 2023 Jan 18;S1569-1993(23)00008-5. doi: 10.1016/j.jcf.2023.01.007. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36669962/
Background: Elexacaftor/tezacaftor/ivacaftor (E/T/I) therapy has resulted in substantial improvements in health status for many with cystic fibrosis. Monitoring of liver tests is recommended due to observed rises in transaminases in trials and cases of hepatotoxicity. Comprehensive data in large populations of unselected individuals and those with established CF related liver disease (CFLD) is lacking.
Methods: Patients prescribed E/T/I at a large, adult centre had liver tests monitored at least 3 monthly for 12 months. Changes in individual liver tests were analysed and abnormalities were compared in those with and without CFLD.
Results: 255 of 267 eligible patients were included. Mild rises in median ALT, AST and bilirubin from baseline to 3 months (all p < 0.001) within normal limits were noted which were sustained. There were no differences in changes in liver tests between those with or without CFLD. There was a significant difference in alkaline phosphatase for those with raised levels at baseline versus those with normal baseline level (-18.5 vs +2.0 IU/L, p = 0.002). Clinically significant rises in ALT and AST occurred in 8 (3.1%) and 6 (2.4%) cases respectively, with derangements in 2 individuals attributed to therapy.
Conclusions: E/T/I leads to a mild, likely clinically insignificant increase in ALT, AST and bilirubin after 3 months which is sustained but does not appear to increase further in the vast majority. Underlying CFLD should not be a barrier to treatment. Although there was a reduction in ALP when elevated at baseline, this was not unique to those with pre-existing CFLD.
Daniel H Tewkesbury is at the Manchester University NHS Foundation Trust, Manchester, UK; Division of Immunology, Immunity to Infection & Respiratory Medicine, University of Manchester, Manchester, UK.
V Terlizzi, S Timpano, M Salvi, A Tosco, A Castaldo, C Fevola, G Leonetti, P Vitullo, A Sepe, R Badolato, D Salvatore. Hyperbilirubinemia and Gilbert’s syndrome in Cystic Fibrosis patients treated with elexacaftor/tezacaftor/ivacaftor. J Cyst Fibros. 2023 Jul 1;S1569-1993(23)00827-5. doi: 10.1016/j.jcf.2023.06.013. Online ahead of print.https://www.cysticfibrosisjournal.com/article/S1569-1993(23)00827-5/fulltext

Vito Terlizzi
ResearchGate
This letter reports a multicenter retrospective study to investigate cases of hyperbilirubinemia following the initiation of ETI therapy (100 mg elexacaftor, 50 mg tezacaftor, and 75 mg ivacaftor co-packaged with a tablet containing ivacaftor 150 mg) in patients with CF at six Italian CF centres. They also evaluated possible concomitant increases in hemoglobin (Hb). The table in the letter summarizes the blood parameters, changes in Forced Expiratory Volume in the 1st second (FEV1), body mass index (BMI), and sweat chloride (SC) before and after three months of ETI therapy.
Seventeen patients were affected among the 503 CF patients on ETI therapy, 52 (10.3%). They developed hyperbilirubinemia after three months of treatment and were investigated for the underlying causes. Seventeen of them (eight adults; mean age: 19.4 years, range 11–32) were tested for Gilbert’s syndrome and resulted homozygous (n=16) or heterozygous (n=1) for UDP-glucuronosyltransferase (UGT1A1)
In conclusion, the study emphasizes the need to consider coexisting Gilbert’s syndrome in cases of unconjugated hyperbilirubinemia to prevent unwarranted discontinuation of ETI treatment. However, it’s important to acknowledge that our retrospective study may be influenced by regional or population-specific factors. Furthermore, the absence of a control group limits our ability to attribute the bilirubin increase solely to ETI therapy. Long-term follow-up and further research are required to establish causality and gain a better understanding of the underlying mechanisms.
Dr V Terlizzi is at the Department of Paediatric Medicine, Cystic Fibrosis Regional Reference Center, Meyer Children’s Hospital IRCCS, Florence, Italy.

