VX-445 (ELEXACAFTOR)/TEZACAFTOR/IVACAFTOR
“TRIKAFTA” (USA) “KAFTRIO” (UK)
2018
2018 Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL; VX16-445-001 Study Group. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles N Engl J Med. 2018 Oct 25;379(17):1612-1620. doi: 10.1056/NEJMoa1807120. Epub 2018 Oct 18. [Pubmed]

Dominic Keating
VX-445 is a next-generation cystic fibrosis transmembrane conductance regulator (CFTR) corrector designed to restore Phe508del CFTR protein function in patients with cystic fibrosis when administered with tezacaftor and ivacaftor (VX-445-tezacaftor-ivacaftor). The authors evaluated the effects of VX-445-tezacaftor-ivacaftor on Phe508del CFTR protein processing, trafficking, and chloride transport in human bronchial epithelial cells. On the basis of in vitro activity, a randomized, placebo-controlled, double-blind, dose-ranging, phase 2 trial was conducted to evaluate oral VX-445-tezacaftor-ivacaftor in patients heterozygous for the Phe508del CFTR mutation and a minimal-function mutation (Phe508del-MF) and in patients homozygous for the Phe508del CFTR mutation (Phe508del-Phe508del) after tezacaftor-ivacaftor run-in. Primary end points were safety and absolute change in percentage of predicted forced expiratory volume in 1 second (FEV1) from baseline.
Results. In vitro, VX-445-tezacaftor-ivacaftor significantly improved Phe508del CFTR protein processing, trafficking, and chloride transport to a greater extent than any two of these agents in dual combination. In patients with cystic fibrosis, VX-445-tezacaftor-ivacaftor had an acceptable safety and side-effect profile. Most adverse events were mild or moderate. The treatment also resulted in an increased percentage of predicted FEV1 of up to 13.8 points in the Phe508del-MF group (P<0.001). In patients in the Phe508del-Phe508del group, who were already receiving tezacaftor-ivacaftor, the addition of VX-445 resulted in an 11.0-point increase in the percentage of predicted FEV1 (P<0.001). In both groups, there was a decrease in sweat chloride concentrations and improvement in the respiratory domain score on the Cystic Fibrosis Questionnaire-Revised.
The authors concluded the use of VX-445-tezacaftor-ivacaftor to target Phe508del CFTR protein resulted in increased CFTR function in vitro and translated to improvements in patients with cystic fibrosis with one or two Phe508del alleles. This approach has the potential to treat the underlying cause of cystic fibrosis in approximately 90% of patients. (Clinical trials NCT03227471 ; and EudraCT number, 2017-000797-11)
Dominic T Keating is Respiratory Physician at the Alfred Hospital · Department of Allergy, Immunology & Respiratory Medicine (AIRmed) Australia, Melbourne
2019
Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, Ramsay BW, Taylor0Cousar JL, Tullis E, Vermeulen F, marifwada G, Mosowski SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Jain Rassha for the VX17-445-i102 Study Group. Elexacaftor-tezacaftor-iavacaftor for cystic fibrosis with a single The 508del allele. N Eng J Med 2019; 381:1809-19. [Pubmed]

Peter Middleton
Cystic fibrosis is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein, and nearly 90% of patients have at least one copy of the Phe508del CFTR mutation. In a phase 2 trial involving patients who were heterozygous for the Phe508del CFTR mutation and a minimal- function mutation (Phe508del–minimal function genotype), the next-generation CFTR corrector elexacaftor, in combination with tezacaftor and ivacaftor, improved Phe508del CFTR function and clinical outcomes.
The authors conducted a phase 3, randomised, double-blind, placebo-controlled trial to confirm the efficacy and safety of elexacaftor–tezacaftor–ivacaftor in patients 12 years of age or older with cystic fibrosis with Phe508del–minimal function geno- types. Patients were randomly assigned to receive elexacaftor–tezacaftor–ivacaftor or placebo for 24 weeks. The primary end point was absolute change from baseline in percentage of predicted forced expiratory volume in 1 second (FEV1) at week 4.
A total of 403 patients underwent randomisation and received at least one dose of active treatment or placebo. Elexacaftor–tezacaftor–ivacaftor, relative to placebo, resulted in a percentage of predicted FEV1 that was 13.8 points higher at 4 weeks and 14.3 points higher through 24 weeks, a rate of pulmonary exacerbations that was 63% lower, a respiratory domain score on the Cystic Fibrosis Questionnaire– Revised (range, 0 to 100, with higher scores indicating a higher patient-reported quality of life with regard to respiratory symptoms; minimum clinically important difference, 4 points) that was 20.2 points higher, and a sweat chloride concentration that was 41.8 mmol per litre lower (P<0.001 for all comparisons). Elexacaftor– tezacaftor–ivacaftor was generally safe and had an acceptable side-effect profile. Most patients had adverse events that were mild or moderate. Adverse events leading to discontinuation of the trial regimen occurred in 1% of the patients in the elexacaftor–tezacaftor–ivacaftor group.
Conclusion – Elexacaftor–tezacaftor–ivacaftor was efficacious in patients with cystic fibrosis with Phe508del–minimal function genotypes, in whom previous CFTR modulator regimens were ineffective. (VX17-445-102 ClinicalTrials.gov number, NCT03525444.)
Professor Peter Middleton is Clinical Professor of Respiratory and Sleep Medicine Westmead Hospital and CF Research Group, Ludwig Engel Centre for Respiratory Research, University of Sydney.
Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, Moskowitz SM, Waltz D, Sosnay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy KS; VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Collaborators (45). Lancet. 2019 Oct 30. pii: S0140-6736(19)32597-8. doi: 10.1016/S0140-6736(19)32597-8. [Epub ahead of print] Full copy available [Pubmed]

Harry Heijerman
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators correct the basic defect caused by CFTR mutations. Improvements in health outcomes have been achieved with the combination of a CFTR corrector and potentiator in people with cystic fibrosis homozygous for the F508del mutation. The addition of elexacaftor (VX-445), a next-generation CFTR corrector, to tezacaftor plus ivacaftor further improved F508del-CFTR function and clinical outcomes in a phase 2 study in people with cystic fibrosis homozygous for the F508del mutation.
This phase 3, multicentre, randomised, double-blind, active-controlled trial of elexacaftor in combination with tezacaftor plus ivacaftor was done at 44 sites in four countries. Eligible participants were those with cystic fibrosis homozygous for the F508del mutation, aged 12 years or older with stable disease, and with a percentage predicted forced expiratory volume in 1 s (ppFEV1) of 40-90%, inclusive. After a 4-week tezacaftor plus ivacaftor run-in period, participants were randomly assigned (1:1) to 4 weeks of elexacaftor 200 mg orally once daily plus tezacaftor 100 mg orally once daily plus ivacaftor 150 mg orally every 12 h versus tezacaftor 100 mg orally once daily plus ivacaftor 150 mg orally every 12 h alone. The primary outcome was the absolute change from baseline (measured at the end of the tezacaftor plus ivacaftor run-in) in ppFEV1 at week 4. Key secondary outcomes were absolute change in sweat chloride and Cystic Fibrosis Questionnaire-Revised respiratory domain (CFQ-R RD) score. This study is registered with ClinicalTrials.gov, NCT03525548.
Between Aug 3 and Dec 28, 2018, 113 participants were enrolled. Following the run-in, 107 participants were randomly assigned (55 in the elexacaftor plus tezacaftor plus ivacaftor group and 52 in the tezacaftor plus ivacaftor group) and completed the 4-week treatment period. The elexacaftor plus tezacaftor plus ivacaftor group had improvements in the primary outcome of ppFEV1 (least squares mean [LSM] treatment difference of 10·0 percentage points [95% CI 7·4 to 12·6], p<0·0001) and the key secondary outcomes of sweat chloride concentration (LSM treatment difference -45·1 mmol/L [95% CI -50·1 to -40·1], p<0·0001), and CFQ-R RD score (LSM treatment difference 17·4 points [95% CI 11·8 to 23·0], p<0·0001) compared with the tezacaftor plus ivacaftor group. The triple combination regimen was well tolerated, with no discontinuations. Most adverse events were mild or moderate; serious adverse events occurred in two (4%) participants receiving elexacaftor plus tezacaftor plus ivacaftor and in one (2%) receiving tezacaftor plus ivacaftor.
The authors concluded Elexacaftor plus tezacaftor plus ivacaftor provided clinically robust benefit compared with tezacaftor plus ivacaftor alone, with a favourable safety profile, and shows the potential to lead to transformative improvements in the lives of people with cystic fibrosis who are homozygous for the F508del mutation.
Dr Harry Heijerman is at the Department of Pulmonology, University Medical Center Utrecht, Utrecht, Netherlands.
Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, McKee CM, Waltz D, Moskowitz SM, Savage J, Xuan F, Rowe SM.Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles.ERJ Open Res. 2019 Jun 17;5(2). pii: 00082-2019. doi: 10.1183/23120541.00082-2019. eCollection 2019 Apr. [Pubmed] Free PMC Article

Jennifer Taylor-Cousar
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator gene (CFTR) that result in diminished quantity and/or function of the CFTR anion channel. F508del-CFTR, the most common CF-causing mutation (found in ∼90% of patients), causes severe processing and trafficking defects, resulting in decreased CFTR quantity and function. CFTR modulators are medications that increase the amount of mature CFTR protein (correctors) or enhance channel function (potentiators) at the cell surface. Combinations of CFTR correctors and potentiators (i.e. lumacaftor/ivacaftor, tezacaftor/ivacaftor) have demonstrated clinical benefit in subsets of patients. However, none are approved for patients with CF heterozygous for F508del-CFTR and a minimal function mutation, i.e. a mutation that produces either no protein or protein that is unresponsive to currently approved CFTR modulators. Next-generation CFTR correctors VX-659 and VX-445, each in triple combination with tezacaftor and ivacaftor, improve CFTR processing, trafficking and function in vitro and have demonstrated clinical improvements in phase 2 studies in patients with CF with one or two F508del–CFTR alleles. Here, we present the rationale and design of four randomised phase 3 studies, and their open-label extensions, evaluating VX-659 (ECLIPSE) or VX-445 (AURORA) plus tezacaftor and ivacaftor in patients with one or two F508del–CFTR alleles.
Corresponding author Dr Steven Rowe, Gregory Flemming James Cystic Fibrosis Research Center, University of Alabama at Birmingham.A very comprehensive review of triple-combination CFTR modulators
Hoy SM. Elexacaftor/Ivacaftor/Tezacaftor: First Approval. Drugs. 2019 Nov 29. doi: 10.1007/s40265-019-01233-7. [Epub ahead of print]Author information.[Pubmed]
A fixed-dose combination tablet of the cystic fibrosis transmembrane conductance regulator (CFTR) corrector tezacaftor and the CFTR potentiator ivacaftor with the next-generation CFTR corrector elexacaftor (hereafter referred to as elexacaftor/ivacaftor/tezacaftor) [Trikafta™] has been developed by Vertex Pharmaceuticals Inc. to treat patients with the most common cystic fibrosis mutation (F508del). Its use has been associated with statistically significant and/or clinically meaningful improvements in lung function and respiratory-related quality of life compared with comparator regimens (placebo or ivacaftor/tezacaftor) in multinational phase II and III studies, and in October 2019 elexacaftor/ivacaftor/tezacaftor was approved by the US FDA for the treatment of cystic fibrosis in patients aged ≥ 12 years who have ≥ 1 F508del mutation in the CFTR gene. A regulatory assessment for elexacaftor/ivacaftor/tezacaftor as a treatment for cystic fibrosis is underway in the EU. This article summarises the milestones in the development of elexacaftor/ivacaftor/tezacaftor leading to this first approval for the treatment of cystic fibrosis in patients aged ≥ 12 years who have ≥ 1 F508del mutation in the CFTR gene.
Dr S M Hoy is at Springer Nature, Private Bag 65901, Mairangi Bay, Auckland
2020
New Drug Hailed as Major Breakthrough in Cystic Fibrosis. Am J Med Genet A. 2020 Jan;182(1):8-9. doi: 10.1002/ajmg.a.61420.[Pubmed] This is my summary as their is no abstract: No authors are listed for this neat account of the progress in the development of CFTR modulator therapy from the FDA approval of ivacaftor (VX-771) in January 2012 for patients with one copy of the G551D mutation, to Orkambi, the combination of lumacaftor (VX-809) and ivacaftor, for those with 2 copies of the F508del mutation in 2015, to another drug combination of tezacaftor (VX-661) and ivacaftor (Symdeko) in 2018 for those people with at least one F508del mutation.
In 2019 the combination drug Trikafta, combining elexacaftor (VX-445), ivacaftor (Kalydeco) and tezacaftor (VX-661), was approved for patients aged 12 years and over with at least one F508del – a group comprising some 90% of the CF population. Approval was based on two phase III clinical trials (Middleton et al, 2019; Heijerman et al, 2019). Sadly these drugs are very expensive and funding is and will remain a major problem.
Cuevas-Ocaña S, Laselva O, Avolio J, Nenna R. The era of CFTR modulators: improvements made and remaining challenges Breathe (Sheff). 2020 Jun;16(2):200016. doi: 10.1183/20734735.0016-2020. [Pubmed] PMC article.

Sara Cuevas-Ocaña
The entry into the clinic of CFTR modulators such as TRIKAFTA has significantly improved life for 90% CF patients carrying one or two F508del mutations but challenges remain for rare CFTR mutations and the management of lung infections.
This last decade has created historical moments for CF, primarily driven by the development of CFTR modulators. First for patients with gating mutations who benefited from Kalydeco, then for those patients with one F508del copy who could benefit from Orkambi, and most recently, patients with at least one F508del copy who can benefit from Trikafta. Despite heterogeneity in patient response, the majority of CF patients will be greatly impacted by using a CFTR modulator therapy, thus changing the trajectory of their life. Furthermore, it remains to be determined whether the next generation of modulators will be effective for individuals bearing rare mutations that are Orkambi resistant. However, it should not be forgotten that there still remains 10% of the CF population who do not have a targeted CFTR modulator treatment. In addition, even with these novel drug therapies, managing infections will continue to be a challenge, thus the CF community will need to adapt the standards for an improving, but ageing CF population.
Dr Sara Cuevas-Ocaña is a Research Fellow in the University of Nottingham Biodiscovery Institute, Nottingham, UK
– An excellent very readable summary of the present situation regarding the modulators including the key references
Elizabeth Marie Gavioli, Nerli Guardado, Farah Haniff, Nouran Deiab, Etty Vider. A current review of the safety of cystic fibrosis transmembrane conductance regulator modulators. J Clin Pharm Ther 2020 Dec 7.doi: 10.1111/jcpt.13329. Online ahead of print. [Pubmed]
What is known and objective: Treatment with cystic fibrosis transmembrane conductance regulator (CFTR) modulators has led to improved clinical outcomes and an increase in lifespans of cystic fibrosis (CF) patients. As CF patients continue to live longer, they are at risk for developing adverse drug reactions associated with polypharmacy and CFTR modulators.
Comment: The authors aim to describe safety concerns of the current combination CFTR modulators, based upon a literature review, including notable safety concerns and recommendations for drug-drug interactions.
What is new and conclusion: Cystic fibrosis transmembrane conductance regulator agents are generally well tolerated with low discontinuation rates when compared to placebo. Elevations in liver enzymes and drug-drug interactions are the most notable safety concerns. Additionally, lumacaftor/ivacaftor has shown more respiratory-related adverse events and drug-drug interactions compared to elexacaftor/tezacaftor/ivacaftor and tezacaftor/ivacaftor. Postmarketing studies are needed to determine long-term safety concerns.
Elizabeth Marie Gavioli is assistant professor of pharmacy practice at the Arnold and Marie Schwartz College of Pharmacy and Health Sciences, Brooklyn, NY, USA.
DiMango E, Overdevest J, Keating C, Francis SF, Dansky D, Gudis D. Effect of highly effective modulator treatment on sinonasal symptoms in cysticfibrosis. J Cyst Fibros. 2020 Jul 18:S1569-1993(20)30794-3. doi: 10.1016/j.jcf.2020.07.002. Online ahead of print. [Pubmed]

Emily DiMango
Elexacaftor-tezacaftor-ivacaftor is a highly effective modulator for cystic fibrosis (CF) patients homozygous or heterozygous for F508del. Effects of the drug on sinonasal symptoms have not been studied Adult participants were prospectively evaluated at baseline and after three months of treatment using validated questionnaires assessing sinonasal symptoms (SNOT-22) and CF-related quality of life (CFQ-R).
Results: Forty-three participants completed the study; 23 were taking other CF transmembrane conductance (CFTR) modulators at the time of study participation. There was a significant improvement in mean SNOT-22 from 34.8 (29.4-40, 95% confidence interval) to 24.4 (19.9-29.0) (p = 0.000003) and in the Respiratory domain of the CFQR from 60.6 (57.1-64.1) to 83.3 (79.4-87.2) (p = 0.0000002), both achieving a minimal clinically important difference. Patients previously taking CFTR modulators experienced a greater benefit in sinonasal and respiratory symptoms
Conclusions: Elexacaftor-tezacaftor-ivacaftor is associated with significant improvement in sinonasal symptoms; previous use of CFTR modulators is associated with greater benefit
Dr Emily DiMango is Professor of Medicine at Columbia University Medical Center; Director, John Edsall-John Wood Asthma Center; Director, Adult Cystic Fibrosis Program
Onofrio Laselva, Claire Bartlett, Tarini N A Gunawardena, Hong Ouyang, Paul D W Eckford, Theo J Moraes , Christine E Bear , Tanja Gonska. Rescue of multiple class II CFTR mutations by elexacaftor+ tezacaftor+ivacaftor mediated in part by the dual activities of Elexacaftor as both corrector and potentiator. Eur Respir J 2020 Dec 10;2002774.doi: 10.1183/13993003.02774-2020. Online ahead of print.
Positive results in preclinical studies of the triple combination of elexacaftor, tezacaftor and ivacaftor, performed in airway epithelial cell cultures obtained from patients harboring F508del-CFTR, translated to impressive clinical outcomes for subjects carrying this mutation in clinical trials and approval of TRIKAFTATM Encouraged by this correlation, we were prompted to evaluate the effect of the elexacaftor, tezacaftor and ivacaftor triple combination on primary nasal epithelial cultures obtained from individuals with rare Class II cystic fibrosis causing mutations; G85E, M1101K and N1303K for which TRIKAFTATM is not approved. Cultures from individuals homozygous for M1101K responded better than cultures harboring G85E and N1303K after treatment with the triple combination with respect to improvement in regulated channel function and protein processing. A similar genotype specific effect of the triple combination was observed when the different mutations were expressed in HEK-293 cells, supporting the hypothesis that these modulators may act directly on the mutant proteins. Detailed studies in nasal cultures and HEK-293 cells showed that the corrector: elexacaftor, exhibited dual activity as both corrector and potentiator and suggested that the potentiator activity contributes to its pharmacological activity. These preclinical studies using nasal epithelial cultures identified mutation genotypes for which elexacaftor, tezacaftor and ivacaftor may produce clinical responses that are comparable to, or inferior to those observed for F508del-CFTR.
Onofrio Laselva is with the Programme in Molecular Medicine, Hospital for Sick Children, Toronto, Canada and the Department of Physiology University of Toronto
Scott A Kanner, Zunaira Shuja, Papiya Choudhury, Ananya Jain, Henry M Colecraft. Targeted deubiquitination rescues distinct trafficking-deficient ion channelopathies. Nat Methods 2020 Dec;17(12):1245-1253.doi: 10.1038/s41592-020-00992-6. Epub 2020 Nov 9. [Pubmed]

Scott Kanner
Impaired protein stability or trafficking underlies diverse ion channelopathies and represents an unexploited unifying principle for developing common treatments for otherwise dissimilar diseases. Ubiquitination limits ion channel surface density, but targeting this pathway for the purposes of basic study or therapy is challenging because of its prevalent role in proteostasis. We developed engineered deubiquitinases (enDUBs) that enable selective ubiquitin chain removal from target proteins to rescue the functional expression of disparate mutant ion channels that underlie long QT syndrome (LQT) and cystic fibrosis (CF). In an LQT type 1 (LQT1) cardiomyocyte model, enDUB treatment restored delayed rectifier potassium currents and normalized action potential duration. CF-targeted enDUBs synergistically rescued common (ΔF508) and pharmacotherapy-resistant (N1303K) CF mutations when combined with the US Food and Drug Administation (FDA)- approved drugs Orkambi (lumacaftor/ivacaftor) and Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor). Altogether, targeted deubiquitination via enDUBs provides a powerful protein stabilization method that not only corrects diverse diseases caused by impaired ion channel trafficking, but also introduces a new tool for deconstructing the ubiquitin code in situ
Scott A Kanner is on the Doctoral Program in Neurobiology and Behavior, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, USA.
Onofrio Laselva, Jacqueline McCormack, Claire Bartlett, Wan Ip, Tarini N A Gunawardena, Hong Ouyang, Paul D W Eckford, Tanja Gonska, Theo J Moraes, Christine E Bear. Preclinical Studies of a Rare CF-Causing Mutation in the Second Nucleotide Binding Domain (c.3700A>G) Show Robust Functional Rescue in Primary Nasal Cultures by Novel CFTR Modulators. J Pers Med 2020 Nov 5;10(4):209.doi: 10.3390/jpm10040209. Free PMC article [Pubmed]

Onofrio Laselva
The combination therapies ORKAMBITM and TRIKAFTATM are approved for people who have the F508del mutation on at least one allele. In this study we examine the effects of potentiator and corrector combinations on the rare mutation c.3700A>G. This mutation produces a cryptic splice site that deletes six amino acids in NBD2 (I1234-R1239del). Like F508del it causes protein misprocessing and reduced chloride channel function. We show that a novel cystic fibrosis transmembrane conductance regulator CFTR modulator triple combination (AC1, corrector, AC2-2, co-potentiator and AP2, potentiator), rescued I1234-R1239del-CFTR activity to WT-CFTR level in HEK293 cells. Moreover, we show that although the response to ORKAMBI was modest in nasal epithelial cells from two individuals homozygous for I1234-R1239del-CFTR, a substantial functional rescue was achieved with the novel triple combination. Interestingly, while both the novel CFTR triple combination and TRIKAFTATM treatment showed functional rescue in gene-edited I1234-R1239del-CFTR-expressing HBE cells and in nasal cells from two CF patients heterozygous for I1234-R1239del/W1282X, nasal cells homozygous for I1234-R1239del-CFTR showed no significant response to the TRIKAFTATMcombination. These data suggest a potential benefit of CFTR modulators on the functional rescue of I1234-R1239del -CFTR, which arises from the rare CF-causing mutation c.3700A>G, and highlight that patient tissues are crucial to our full understanding of functional rescue in rare CFTR mutations.
Onofrio Laselva at the Programme in Molecular Medicine, Hospital for Sick Children, Toronto, ON M5G 8X4, Canada and the Department of Physiology, University of Toronto, Toronto, ON M5G 8X4, Canada

Guido Veit
Guido Veit, Ariel Roldan, Mark A Hancock, Dillon F Da Fonte, Haijin Xu, Maytham Hussein, Saul Frenkiel, Elias Matouk, Tony Velkov, Gergely L Lukacs . Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight 2020 Sep 17;5(18):e139983.doi: 10.1172/jci.insight.139983. Free PMC article [Pubmed]
Based on its clinical benefits, Trikafta – the combination of folding correctors VX-661 (tezacaftor), VX-445 (elexacaftor), and the gating potentiator VX-770 (ivacaftor) – was FDA approved for treatment of patients with cystic fibrosis (CF) carrying deletion of phenylalanine at position 508 (F508del) of the CF transmembrane conductance regulator (CFTR) on at least 1 allele.
Neither the mechanism of action of VX-445 nor the susceptibility of rare CF folding mutants to Trikafta are known. Here, we show that, in human bronchial epithelial cells, VX-445 synergistically restores F508del-CFTR processing in combination with type I or II correctors that target the nucleotide binding domain 1 (NBD1) membrane spanning domains (MSDs) interface and NBD2, respectively, consistent with a type III corrector mechanism. This inference was supported by the VX-445 binding to and unfolding suppression of the isolated F508del-NBD1 of CFTR. The VX-661 plus VX-445 treatment restored F508del-CFTR chloride channel function in the presence of VX-770 to approximately 62% of WT CFTR in homozygous nasal epithelia. Substantial rescue of rare misprocessing mutations (S13F, R31C, G85E, E92K, V520F, M1101K, and N1303K), confined to MSD1, MSD2, NBD1, and NBD2 of CFTR, was also observed in airway epithelia, suggesting an allosteric correction mechanism and the possible application of Trikafta for patients with rare misfolding mutants of CFTR.
Dr Guido Veit is in the Department of Physiology and.2SPR-MS Facility, McGill University, Montréal, Quebec, Canada.
William Tindell, Amanda Su, Sarah M Oros, Abner O Rayapati, Gopalkumar Rakesh. Trikafta and Psychopathology in Cystic Fibrosis: A Case Report. Psychosomatics Nov-Dec 2020;61(6):735-738.doi: 10.1016/j.psym.2020.06.021. Epub 2020 Jul 2. [Pubmed]

William Tindell
Abstract requested but not available.
Dr William Tindell is a resident in the Department of Psychiatry, University of Kentucky Medical Center, Lexington, KY
Nazan Çobanoğlu, Uğur Özçelik, Erkan Çakır, Tuğba Şişmanlar Eyüboğlu, Sevgi Pekcan, Güzin Cinel.et al. Patients eligible for modulator drugs: Data from cystic fibrosis registry of Turkey. Pediatr Pulmonol 2020 Sep;55(9):2302-2306.doi: 10.1002/ppul.24854. Epub 2020 May 26.[Pubmed]

Nazan Cobanoglu
Background: A better understanding of cystic fibrosis transmembrane conductance regulator biology has led to the development of modulator drugs such as ivacaftor, lumacaftor-ivacaftor, tezacaftor-ivacaftor, and elexacaftor-tezacaftor-ivacaftor. This cross-sectional study evaluated cystic fibrosis (CF) patients eligible for modulator drugs.
Methods: Data for age and genetic mutations from the Cystic Fibrosis Registry of Turkey collected in 2018 were used to find out the number of patients who are eligible for modulator therapy.
Results: Of registered 1488 CF patients, genetic analysis was done for 1351. The numbers and percentages of patients and names of the drugs, that the patients are eligible for, are as follows: 122 (9.03%) for ivacaftor, 156 (11.54%) for lumacaftor-ivacaftor, 163 (11.23%) for tezacaftor-ivacaftor, and 57 (4.21%) for elexacaftor-tezacaftor-ivacaftor. Among 1351 genotyped patients total of 313 (23.16%) patients are eligible for currently licensed modulator therapies (55 patients were shared by ivacaftor and tezacaftor-ivacaftor, 108 patients were shared by lumacaftor-ivacaftor and tezacaftor-ivacaftor, and 22 patients were shared by tezacaftor-ivacaftor and elexacaftor-tezacaftor-ivacaftor groups).
Conclusions: The present study shows that approximately one-fourth of the registered CF patients in Turkey are eligible for modulator drugs. As, frequent mutations that CF patients have in Turkey are different from North American developing countries like Turkey is noteworthy and European CF patients, developing modulator drugs effective for those mutations is necessary. Furthermore, as modulator drugs are very expensive currently, financial support of the government in developing countries like Turkey is noteworthy
Prof. Dr Fatama Nazan Çobanoğlu is at the Division of Pediatric Pulmonology, Faculty of Medicine, Ankara University, Ankara, Turkey.
Shannon M Rotolo, Stephanie Duehlmeyer, Sarah M Slack, Hollyann R Jacobs, Brian Heckman. Testicular pain following initiation of elexacaftor/tezacaftor/ivacaftor in males with cystic fibrosis. J Cyst Fibros 2020 Sep;19(5):e39-e41.doi: 10.1016/j.jcf.2020.04.017.Epub 2020 May 27. [Pubmed]

Shannon Rotolo
There were no documented reports of testicular pain during clinical trials. In this case series, we discuss 7 males between 17 and 39 years of age who reported testicular pain or discomfort within the first two weeks of starting therapy. The precise mechanism of this side effect is unknown, but it may be related to restoration of CFTR function in the male reproductive tract. All patients experienced resolution of this side effect within a week after onset, regardless of the management, except for one. Further research is needed to determine short- and long-term impact of this drug on male fertility. Until more data is available, the authors recommend counselling patients on contraceptive options.
Shannon M Rotolo is Clinical Pharmacy Specialist at the University of Chicago Medicine, Department of Pharmacy, Chicago, IL
New Drug Hailed as Major Breakthrough in Cystic Fibrosis. Am J Med Genet A. 2020 Jan;182(1):8-9. doi: 10.1002/ajmg.a.61420.[Pubmed] My summary as their is no abstract: No authors are listed for this neat account of the progress in the development of CFTR modulator therapy from the FDA approval of ivacaftor (VX-771) in January 2012 for patients with one copy of the G551D mutation, to Orkambi, the combination of lumacaftor (VX-809) and ivacaftor, for those with 2 copies of the F508del mutation in 2015, to another drug combination of tezacaftor (VX-661) and ivacaftor (Symdeko) in 2018 for those people with at least one F508del mutation.
In 2019 the combination drug Trikafta, combining elexacaftor (VX-445), ivacaftor (Kalydeco) and tezacaftor (VX-661), was approved for patients aged 12 years and over with at least one F508del – a group comprising some 90% of the CF population. Approval was based on two phase III clinical trials (Middleton et al, 2019; Heijerman et al, 2019). Sadly these drugs are very expensive and funding is and will remain a major problem.
Giada Righetti, Monica Casale, Michele Tonelli, Nara Liessi , Paola Fossa, Nicoletta Pedemonte, Enrico Millo , Elena Cichero. New Insights into the Binding Features of F508del CFTR Potentiators: A Molecular Docking, Pharmacophore Mapping and QSAR Analysis Approach. Pharmaceuticals (Basel) 2020 Dec 4;13(12):E445.doi: 10.3390/ph13120445. [Pubmed]

Giada Righetti
Cystic fibrosis (CF) is the autosomal recessive disorder most recurrent in Caucasian populations. To combat this disease, many life-prolonging therapies are required and deeply investigated, including the development of the so-called cystic fibrosis transmembrane conductance regulator (CFTR) modulators, such as correctors and potentiators. Combination therapy with the two series of drugs led to the approval of several multi-drug effective treatments, such as Orkambi, and to the recent promising evaluation of the triple-combination Elexacaftor-Tezacaftor-Ivacaftor.
This scenario enlightened the effectiveness of the multi-drug approach to pave the way for the discovery of novel therapeutic agents to contrast CF. The recent X-crystallographic data about the human CFTR in complex with the well-known potentiator Ivacaftor (VX-770) opened the possibility to apply a computational study aimed to explore the key features involved in the potentiator binding.
Herein, we discussed molecular docking studies performed onto the chemotypes so far discussed in the literature as CFTR potentiator, reporting the most relevant interactions responsible for their mechanism of action, involving Van der Waals interactions and π-π stacking with F236, Y304, F305 and F312, as well as H-bonding F931, Y304, S308 and R933.
This kind of positioning will stabilize the effective potentiator at the CFTR channel. These data have been accompanied by pharmacophore analyses, which promoted the design of novel derivatives endowed with a main (hetero)aromatic core connected to proper substituents, featuring H-bonding moieties. A highly predictive quantitative-structure activity relationship (QSAR) model has been developed, giving a cross-validated r2 (r2cv) = 0.74, a non-cross validated r2 (r2ncv) = 0.90, root mean square error (RMSE) = 0.347, and a test set r2 (r2pred) = 0.86.
On the whole, the results are expected to gain useful information to guide the further development and optimization of new CFTR potentiators
Smyth RL. New drug treatments for cystic fibrosis. BMJ. 2020 Jan 20;368:m118. doi: 10.1136/bmj.m11 [Pubmed]

Rosiland Smyth
The present situation regarding the new CFTR modulators is neatly summarised by Prof. Ros Smyth in this BMJ article. As some readers may not have access to the full text, I have summarised some of the key points and added the key references –
Mutations that result in CFTR being expressed on the cell surface but incorrectly regulated, such as G551D, were the most straightforward targets. Ivacaftor, a potentiator, increases the time that the CFTR chloride channel remains open. In a phase III randomised placebo controlled trial, ivacaftor improved forced expiratory volume in one second (FEV1) by 11% as well as respiratory symptoms and weight gain without substantial adverse effects (Ramsey et al 2011). The trial recruited 161 patients and was completed in 19 months despite the small pool of just 2000 people worldwide with the G551D mutation. Ivacaftor was licensed in Europe in 2012.
Ivacaftor is effective for only 4-5% of patients with cystic fibrosis. Many more patients have the F508del mutation, which causes misfolding of CFTR protein so that it remains trapped in the cell’s endoplasmic reticulum. Any CFTR that does reach the cell surface is unable to activate normally. Although potentiators can enhance activation at the cell surface, dealing with the trapped protein requires agents that correct the misfolding (correctors).
In 2015, a trial of Orkambi, a drug combining the corrector lumacaftor with the potentiator ivacaftor, showed improvements in weight gain and respiratory exacerbations among patients who were homozygous for F508del, but the modest 3% increase in FEV1 relative to placebo was associated with transient dyspnoea and abnormal liver function (Wainwright et al, 2015)
Subsequent trials of a different corrector, tezacaftor, combined with ivacaftor showed similar improvements in FEV1 to Orkambi but with fewer side effects (Taylor-Cousar et al, 2017; Rowe SM et al, 2017)
In 2018, tezacaftor-ivacaftor (Symkevi) was licensed in the US and Europe for patients with F508del (either homozygous or F508del plus an allele with another mutation associated with residual CFTR function), representing around 50% of patients worldwide (Davies JC et al, 2018; Keating D et al, 2018).
Although 90% of people with cystic fibrosis have at least one copy of the F508del mutation, around 30% also have other minimal function mutations that are unresponsive to current CFTR modulators. This led to the development of next generation correctors and trials of “triple therapy” combining two correctors and a potentiator.
In 2019, phase III trials of elexacaftor-ivacaftor-tezacaftor in patients with F508del reported greater than 10% improvements in FEV1 compared with placebo and substantial reductions in respiratory exacerbations (Heijerman et al, 2019; Middleton et al, 2019).
– Ramsey BW, Davies J, McElvaney NG, et al., VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72. doi:10.1056/NEJMoa1105185 [Pubmed] –Wainwright CE, Elborn JS, Ramsey BW, et al., TRAFFIC Study Group, TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med2015;373:2231.doi:10.1056/NEJMoa1409547 [Pubmed]
– Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med2017;377:2013-23. doi:10.1056/NEJMoa1709846 [Pubmed]
– Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med2017;377:2024-35. doi:10.1056/NEJMoa1709847 [Pubmed]
– Davies JC, Moskowitz SM, Brown C, et al., VX16-659-101 Study Group. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med2018; 379:1599-611. doi:10.1056/NEJMoa1807119 pmid [Pubmed
– Keating D, Marigowda G, Burr L, et al., VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med2018; 379:1612-20. doi:10.1056/NEJMoa1807120 pmidL [Pubmed]
– Heijerman HGM, McKone EF, Downey DG, et al., VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet2019; 394:1940-8. doi:10.1016/S0140-6736(19)32597-8 [Pubmed]
– Middleton PG, Mall MA, Dřevínek P, et al., VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381:1809-19. doi:10.1056/NEJMoa1908639 [Pubmed]
The authors concluded Lumacaftor-ivacaftor was associated with improvement in lung disease and nutritional status in patients who tolerated treatment. Adults who discontinued lumacaftor-ivacaftor, often owing to adverse events, were found at high risk of clinical deterioration.
Shteinberg M, Taylor-Cousar JL. Impact of CFTR modulator use on outcomes in people with severe cystic fibrosis lung disease. Eur Respir Rev. 2020 Mar 20;29(155). pii: 190112. doi: 10.1183/16000617.0112-2019. Print 2020 Mar 31. Free full text [Pubmed]

Michal Shteinberg
Drug compounds that augment the production and activity of the cystic fibrosis (CF) transmembrane regulator (CFTR) have revolutionised CF care. Many adults and some children with CF suffer advanced and severe lung disease or await lung transplantation. While the hope is that these drug compounds will prevent lung damage when started early in life, there is an ongoing need to care for people with advanced lung disease. The focus of this review is the accumulating data from clinical trials and case series regarding the benefits of CFTR modulator therapy in people with advanced pulmonary disease. We address the impact of treatment with ivacaftor, lumacaftor/ivacaftor, tezacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor on lung function, pulmonary exacerbations, nutrition and quality of life. Adverse events of the different CFTR modulators, as well as the potential for drug-drug interactions, are discussed.
Dr Michal Shteinberg is heading the bronchiectasis and adult CF service in the Pulmonology institute and CF Center, Carmel medical Center, Haifa, Israel and a Clinical Assistant Professor in the Faculty of medicine at the Technion, Israel Institute of Technology.
Kevin W Southern, Jared Murphy, Ian P Sinha, Sarah J Nevitt .Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del). Cochrane Database Syst Rev 2020 Dec 17;12:CD010966. doi: 10.1002/14651858.CD010966.pub3. [Pubmed]

Kevin Southern
Authors’ conclusions: There is insufficient evidence that corrector monotherapy has clinically important effects in pwCF with F508del/F508del. Both dual therapies (lumacaftor-ivacaftor, tezacaftor-ivacaftor) result in similar improvements in QoL and respiratory function with lower pulmonary exacerbation rates. Lumacaftor-ivacaftor was associated with an increase in early transient shortness of breath and longer-term increases in blood pressure (not observed for tezacaftor-ivacaftor). Tezacaftor-ivacaftor has a better safety profile, although data are lacking in children under 12 years. In this population, lumacaftor-ivacaftor had an important impact on respiratory function with no apparent immediate safety concerns; but this should be balanced against the blood pressure increase and shortness of breath seen in longer-term adult data when considering lumacaftor-ivacaftor. There is high-quality evidence of clinical efficacy with probably little or no difference in AEs for triple (elexacaftor-tezacaftor-ivacaftor) therapy in pwCF with one or two F508del variants aged 12 years or older. Further RCTs are required in children (under 12 years) and those with more severe respiratory function.
Prof. Kevin Southern is in the Department of Women’s and Children’s Health, University of Liverpool, Liverpool, UK.
– This is a useful up-to-date review and source of reference of these treatments with a full documentation of the various trials.
Sanja Stanojevic, Katarina Vukovojac, Jenna Sykes, Felix Ratjen, Elizabeth Tullis, Anne L Stephenson. Projecting the impact of delayed access to elexacaftor/tezacaftor/ivacaftor for people with Cystic Fibrosis. J Cyst Fibros 2020Aug 5;S1569-1993(20)308092.doi10.1016/j.jcf.2020.07.017.Online ahead of print [Pubmed]

Sanja Stanojevic
Therapies that target the underlying defect in Cystic Fibrosis (CF) will likely impact the future characteristics of the CF population and healthcare utilization. The objectives of this study were to estimate the potential impact of elexacaftor/tezacaftor/ivacaftor on morbidity and mortality, and the impact of delayed access
A microsimulation transition model was applied to Canadian CF Registry data to forecast lung disease severity, pulmonary exacerbations, deaths and transplants to 2030 under three scenarios: 1) no availability of elexacaftor/tezacaftor/ivacaftor, 2) availability in 2021 (‘early’) or 3) availability in 2025 (‘delayed’). Published Phase III data on treatment effects were used to estimate transition rates between disease severity states.
Results: Under specific assumptions regarding disease state and treatment effect applied to the Canadian CF population it is projected that by 2030, early introduction of elexacaftor/tezacaftor/ivacaftor is expected to reduce the number of individuals with severe lung disease by 60% (95% CI 55.3; 63.9), increase the number of individuals with mild lung disease by 18% (95%CI 18.2; 19.0) and reduce the number of pulmonary exacerbations by 19% (95%CI 18.9; 19.5). Earlier introduction of elexacaftor/tezacaftor/ivacaftor could reduce deaths by 15% (95% 13.2; 18.4) and improve the median age of survival by 9.2 years (7.5; 10.8) over a 10-year period. The expected benefits of therapy are cumulative, therefore delayed access to elexacaftor/tezacaftor/ivacaftor will result in preventable health care utilization and deaths.
Conclusions: Delayed access to elexacaftor/tezacaftor/ivacaftor will have a negative impact on lung health and survival in the CF population.
Dr Sanja Stanojevi is at Translational Medicine, Hospital for Sick Children, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Canada; Department of Community Health and Epidemiology, Dalhousie University, Halifax, Canada.
Dr Scott D Sagel is Professor Pediatrics and Pulmonary Medicine at the Department of Pediatrics, Children’s Hospital Colorado and University of Colorado Anschutz Medical Campus, Aurora, Colorado
2021
Kate M O’Shea , Orla M O’Carroll, Catherine Carroll, Brenda Grogan, Anna Connolly, Lynda O’Shaughnessy, Trevor T Nicholson, Charles G Gallagher, Edward F McKone. Efficacy of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease. Eur Respir J. 2021 Feb 25;57(2):2003079.doi: 10.1183/13993003.03079-2020. Print 2021 Feb. [Pubmed]

Kate O’Shea
No abstract but the following abstract form the article – This study shows that ELX/TEZ/IVA improves multiple outcome measures in a small cohort of 14 patients with advanced CF lung disease attending a single centre and that these improvements are similar to those seen in patients with milder disease.
Follow-up measurement dates varied with mean repeat FEV1 at 26.4±4.2 days, mean BMI at 62±35 days and mean SwCl at 64±84 days after ELX/TEZ/IVA initiation. After treatment with ELX/TEX/IVA, FEV1 improved (27.3±7.3% pred versus 36.3±16.5% pred; p<0.0001). BMI also improved (20.7±3.6 versus 22.1±3.4 kg·m−2; p<0.0001). Sweat chloride results were only available for 11 patients, mainly due to insufficient sweat volume despite multiple attempts, but also revealed significant improvement (104.9±15.04 versus 53.6±23.3 mmol·L−1; p<0.0001). Infective exacerbations requiring hospitalisation reduced in frequency (0.28±0.17 exacerbations per month in 12 months prior versus 0.04±0.07 exacerbations per month during follow-up period of 4.9 months; p<0.001)
Most significant were the reduction in requirement for intravenous antibiotic therapy as well as improvements in lung function and sweat chloride. These results were seen in both patients exposed to previous modulator therapies and in those who were CFTR modulator-naïve. There were few significant adverse events. This therapy is expected to improve the disease trajectory for many CF patients with at least one Phe508del mutation and this expectation should also apply to those groups with more advanced disease.
Kate M O’Shea is studying at the School of Medicine, University College Dublin
Dr Orla M O’Carroll is a Respiratory Specialist Registrar in the Department of Respiratory medicine St Vincent’s University Hospital, Dublin
Guido Veit, Christian Vaccarin, Gergely L Lukacs. Elexacaftor co-potentiates the activity of F508del and gating mutants of CFTR. J Cyst Fibros 2021 Mar 25;S1569-1993(21)00087-4.doi: 10.1016/j.jcf.2021.03.011.Online ahead of print. [Pubmed]

Guido Veit
Trikafta, the combination of elexacaftor (VX-445), tezacaftor (VX-661) and ivacaftor (VX-770), was approved for therapy of cystic fibrosis (CF) patients with at least one allele of the CFTR mutation F508del. While the corrector function of VX-445 is well established, here we investigated the putative potentiator activity of VX-445 alone and in combination with VX-770. Acute addition of VX-445 increased the VX-770-potentiated F508del- and G551D-CFTR current by ~24% and >70%, respectively, in human bronchial and nasal epithelia. Combinatorial profiling and cluster analysis of G551D- and G1244E-CFTR channel activation with potentiator pairs indicated a distinct VX-445 mechanism of action that is, at least, additive to previously identified potentiator classes, including the VX-770. Since VX-770 only partially normalizes the G551D-CFTR channel function and adult G551D patients still experience progressive loss of lung function, VX-445+VX-770 combination therapy could provide clinical benefit to CF patients with the G551D and other dual potentiator responsive mutants.
Dr Guido Veit is a Research Associate in the Department of Physiology, McGill University, Montréal, Canada. His main research interest: Epithelial biology with focus on the interplay between extracellular events (cytokines, extracellular matrix) and cellular responses (transmembrane proteins, signalling, transcriptional activation).
Jennifer L Taylor-Cousar, R Jain. Maternal and fetal outcomes following elexacaftor-tezacaftor-ivacaftor use during pregnancy and lactation. J Cyst Fibros. 2021 Mar 21;S1569-1993(21)00055-2.doi: 10.1016/j.jcf.2021.03.006.Online ahead of print. [Pubmed]

Jennifer Taylor-Cousar
There is currently no data in the literature regarding use of Elexacaftor-tezacaftor-ivacaftor (ETI) in pregnant women. Thus, the decision to continue therapy during pregnancy (with the associated unknown fetal impact) versus discontinuing therapy (with the known risk of maternal health decline) is challenging.
Methods: CF Center staff completed an anonymous questionnaire regarding pregnancy and infant outcomes for women who used ETI during pregnancy and/or lactation.
Results: Of 45 ETI-exposed pregnancies reported to date, complications in 2 mothers and in 3 infants (2 born to mothers with poorly controlled diabetes) were rated by clinicians as unknown (possible) or suspected relatedness to ETI use. Two women terminated unplanned pregnancies. Miscarriage rates were consistent with that known in the general U.S.
Population: Five of the six women who discontinued ETI out of concern for unknown foetal risk restarted because of clinical deterioration. No infant cataracts were reported though only two infants were formally evaluated.
Conclusions: In the context of the known increased rate of complications in women with CF and their infants, data from this retrospective survey is reassuring for women who choose to continue ETI during pregnancy. However, a large, multi-centre prospective study is needed to assess impact of use of ETI in pregnancy.
Dr Jennifer L Taylor-Cousar is in the Departments of Medicine and Pediatrics, National Jewish Health, 1400 Jackson Street; J318, Denver, CO 80206.
– Evidence accumulating that there seems to be no serious consequences of taking ETI during pregnancy.
Edith T Zemanick, Jennifer L Taylor-Cousar, Jane Davies, Ronald L Gibson, Marcus A Mall, Edward F McKone et al, A Phase 3 Open-Label Study of ELX/TEZ/IVA in Children 6 Through 11 Years of Age With CF and at Least One F508del Allele. Am J Respir Crit Care Med 2021 Mar 18. doi: 10.1164/rccm.202102-0509OC.Online ahead of print. [Pubmed]

Edith Zermanick
Rationale: Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was shown to be efficacious and safe in patients aged 12 years and older with cystic fibrosis and at least one F508del-CFTR allele, but has not been evaluated in children <12 years of age.
Objectives: To assess the safety, pharmacokinetics, and efficacy of ELX/TEZ/IVA in children 6 through 11 years of age with F508del-minimal function or F508del-F508del genotypes.
Methods: In this 24-week open-label Phase 3 study, children (N=66) weighing <30 kg received 50% of the ELX/TEZ/IVA adult daily dose (ELX 100 mg once daily, TEZ 50 mg once daily, and IVA 75 mg every 12 hours) while children weighing ≥30 kg received the full adult daily dose (ELX 200 mg once daily, TEZ 100 mg once daily, and IVA 150 mg every 12 hours).
Measurements and main results: The primary endpoint was safety and tolerability. The safety and pharmacokinetic profiles of ELX/TEZ/IVA were generally consistent with those observed in older patients. The most common reported adverse events (AEs) included cough, headache, and pyrexia; in most of the children who had AEs, these were mild or moderate in severity. Through Week 24, ELX/TEZ/IVA treatment improved the ppFEV1 (10.2 percentage points; 95% confidence interval [CI], 7.9 to 12.6), Cystic Fibrosis Questionnaire-Revised respiratory domain score (7.0 points; 95% CI, 4.7 to 9.2), lung clearance index2.5 (-1.71 units; 95% CI, -2.11 to -1.30), and sweat chloride (-60.9 mmol/L; 95% CI, -63.7 to -58.2); body mass index-for-age z-score increased over the 24-week treatment period compared to the pre-treatment baselineds, UK
Conclusions: Our results show ELX/TEZ/IVA is safe and efficacious in children 6 through 11 years of age with at least one F508del-CFTR allele, supporting its use in this patient population.
Dr Edith T Zemanick is Associate Professor at the Colorado Anschutz Medical Campus and Children’s Hospital Colorado, Department of Pediatrics, Aurora, Colorado, United States
Cori L Daines, Wayne J Morgan The Future of Highly Effective Modulator Therapy in Cystic Fibrosis.. Am J Respir Crit Care Med 2021 Jun 15;203(12):1453-1455.doi: 10.1164/rccm.202104-0850ED. 33901406 Free PMC article [Pubmed]

Wayne J Morgan

Cori Daines
There is no abstract, but the Free PMC article is a clear commentary on the article of Zemanick ET et al, Am J Respir Crit Care Med 2021;203:1522-1532.(ABOVE)
The success of highly effective modulator therapy (HEMT) in cystic fibrosis (CF) now illustrates two areas of deficiency: the lack of HEMT for younger children and for approximately 10% of the CF population without a qualifying mutation………
At this point, the previous modulators IVA, lumacaftor/IVA, and TEZ/IVA have all been approved for younger ages than the original phase III qualifying studies (11, 12). IVA has recently been approved down to 4 months, whereas lumacaftor/IVA is approved to 2 years and TEZ/IVA is approved to 6 years (13–15). Longer-term data from these extensions show ongoing safety and efficacy even in the youngest patients (16, 17). Previous modulators have been shown to be safe, both in the short and long term, in young children. This knowledge should reaffirm the safety findings in the current study and should prove the appropriateness of moving ELX/TEZ/IVA to the 6- to 11-year-old age group. The CF community has been waiting for the results of this study and to have ELX/TEZ/IVA for children. The goal and expectation will be much better long-term outcomes for people living with CF.
Both authors are in the Department of Pediatrics University of Arizona Tucson, Arizona.
Cori L Daines is a professor of Pediatric Pulmonary Medicine
Wayne J Morgan is Professor of Pediatrics and Physiology Arizona Elks Foundation Chair for Statewide Pediatric Research and Program Director, Pediatric Pulmonary Fellowship Pediatric Pulmonary and Sleep Medicine
Julian Stashower, Patrick Carr, Virginia Miller, Barrett Zlotoff. Novel reaction to new cystic fibrosis medication Trikafta. Clin Case Rep 2021 May 4;9(5):e04116.doi: 10.1002/ccr3.4116.eCollection 2021 May. [Pubmed] Free PMC article
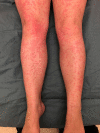
Legs of patient

Julian Stashower
The authors describe a novel case of an urticaria multiforme-type drug reaction to the new cystic fibrosis medication Trikafta (elexacaftor + tezacaftor + ivacaftor). Equipped with this information, clinicians may be more prepared to counsel and treat patients if they experience similar symptoms after beginning Trikafta
There are more images in the Free PMC article
Dr Julian Stashower is at the University of Virginia School of Medicine, Charlottesville USA
This is the first study assessing changes of airway inflammation on a day-to-day basis in CF patients receiving a newly administered CFTR-modulator therapy. It proves a decline of airway inflammation during ivacaftor-therapy.
Dr Jochen G Mainz is Professor at the Cystic Fibrosis Center, Brandenburg Medical School (MHB) University, Klinikum Westbrandenburg, Brandenburg an der Havel,, the CF-Center, Jena University Hospital, Jena and the Faculty of Health Sciences, Joint Faculty of the Brandenburg University of Technology Cottbus -Senftenberg, The Brandenburg Medical School Theodor Fontane and the University of Potsdam, Cottbus, Brandenburg an der Havel and Potsdam, Germany.
C Martin, E Burnet, A Ronayette-Preira, P de Carli, J Martin, L Delmas, B Prieur, P-R Burgel. Patient perspectives following initiation of elexacaftor-tezacaftor-ivacaftor in people with cystic fibrosis and advanced lung disease. Respir Med Res. 2021 May 17;80:100829.doi: 10.1016/j.resmer.2021.100829.Online ahead of print. [Pubmed]
Backgound: Elexacaftor-tezacaftor-ivacaftor partially restores cystic fibrosis transmembrane conductance regulator function, and has been shown to induce significant clinical improvement in patients with at least one Phe508del allele. Yet little data exist on patient perspectives following elexacaftor-tezacaftor-ivacaftor initiation.
Methods: A mixed methods study was conducted using an online 13-item questionnaire (including 9 closed questions and 4 open questions), submitted from July 10th to August 21th2020 to French patients aged 12 years and older with advanced CF who were treated with elexacaftor-tezacaftor-ivacaftor. Their responses were summarized as numbers (%), and free-text items were analysed using a grounded theory approach.
Results: Of 245 patients who started elexacaftor-tezacaftor-ivacaftor in France, 101 (41%) participated. Median [IQR] age was 35 [28-41] years and duration of elexacaftor-tezacaftor-ivacaftor treatment was 4.3 [3.0-5.6] months. Patients generally reported a rapid impact on respiratory symptoms, sleep quality, general well-being and physical self-esteem, and a reduction in overall treatment burden. The majority of patients contrasted treatment burden, symptom severity, depression and a closed future marked by death or transplantation before elexacaftor-tezacaftor-ivacaftor, to renewed and unexpected physical strength, leading to greater self-confidence, autonomy and long-term planning, after treatment initiation. A small number of patients expressed concerns, mainly regarding changes in body representation and/or the fear of becoming dependent on the treatment.
Conclusion: After initiation of elexacaftor-tezacaftor-ivacaftor, CF patients with advanced disease reported rapid and positive physical, psychological and social effects, which translated into improved quality of life and the formulation of new life goals.
Dr C Martin is at the Université de Paris, Institut Cochin, Inserm U1016, Paris, France; Respiratory Medicine and National Reference Cystic Fibrosis Reference Center, Cochin Hospital, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France; ERN-Lung CF network.
Frédéric Becq, Sandra Mirval, Thomas Carrez, Manuella Lévêque, Arnaud Billet, Christelle Coraux, Edouard Sage, Anne Cantereau. The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor. Eur Respir J 2021 Jul 15;2100671.doi: 10.1183/13993003.00671-2021.Online ahead of print. [Pubmed]

Frederic Becq
Trikafta, currently the leading therapeutic in Cystic Fibrosis (CF), has demonstrated a real clinical benefit. This treatment is the triple combination therapy of two folding correctors elexacaftor/tezacaftor (VX445/VX661) plus the gating potentiator ivacaftor (VX770). In this study, our aim was to compare the properties of F508del-CFTR in cells treated with either lumacaftor (VX809), tezacaftor, elexacaftor, elexacaftor/tezacaftor with or without ivacaftor. We studied F508del-CFTR function, maturation and membrane localisation by Ussing chamber and whole-cell patch clamp recordings, Western blot and immunolocalization experiments. With human primary airway epithelial cells and the cell lines CFBE and BHK expressing F508del, we found that, whereas the combination elexacaftor/tezacaftor/ivacaftor was efficient in rescuing F508del-CFTR abnormal maturation, apical membrane location and function, the presence of ivacaftor limits these effects. The basal F508del-CFTR short-circuit current was significantly increased by elexacaftor/tezacaftor/ivacaftor and elexacaftor/tezacaftor compared to other correctors and non-treated cells, an effect dependent on ivacaftor and cAMP. These results suggest that the level of the basal F508del-CFTR current might be a marker for correction efficacy in CF cells. When cells were treated with ivacaftor combined to any correctors, the F508del-CFTR current was unresponsive to the subsequently acute addition of ivacaftor unlike the CFTR potentiators genistein and Cact-A1 which increased elexacaftor/tezacaftor/ivacaftor and elexacaftor/tezacaftor-corrected F508del-CFTR currents. These findings show that ivacaftor reduces the correction efficacy of Trikafta. Thus, combining elexacaftor/tezacaftor with a different potentiator might improve the therapeutic efficacy for treating CF patients
Professor Frédéric Becq is at the Laboratoire Signalisation et Transports Ioniques Membranaires, Université de Poitiers, France.
Marika Comegna , Vito Terlizzi, Donatello Salvatore, Carmela Colangelo, Antonella Miriam Di Lullo, Immacolata Zollo , Giovanni Taccetti, Giuseppe Castaldo, Felice Amato. Elexacaftor-Tezacaftor-Ivacaftor Therapy for Cystic Fibrosis Patients with The F508del/Unknown Genotype. Antibiotics (Basel)2021 Jul 7;10(7):828.doi: 10.3390/antibiotics10070828. Free PMC article [Pubmed]

Marika Comegna
The new CFTR modulator combination, elexacaftor/tezacaftor/ivacaftor (Trikafta) was approved by the FDA in October 2019 for treatment of Cystic Fibrosis in patients 6 years of age or older who have at least one F508del mutation in one allele and a minimal-function or another F508del mutation in the other allele. However, there is a group of patients, in addition to those with rare mutations, in which despite the presence of a F508del in one allele, it was not possible to identify any mutation in the other allele. To date, these patients are excluded from treatment with Trikafta in Italy, where the CF patients carrying F508del/unknown represent about 1.3% (71 patients) of the overall Italian CF patients. In this paper we show that the Trikafta treatment of nasal epithelial cells, derived from F508del/Unknown patients, results in a significant rescue of CFTR activity. Based on our findings, we think that the F508del/Unknown patients considered in this study could obtain clinical benefits from Trikafta treatment, and we strongly suggest their eligibility for this type of treatment. This study, adding further evidence in the literature, once again confirms the validity of functional studies on nasal cells in the cystic fibrosis theratyping and personalized medicine
Dr Marika Comegna is in the Department of Molecular Medicine and Medical Biotechnologies, University of Naples Federico II, Via Pansini, 5, 80131 Naples, Italy.
CEINGE-Advanced Biotechnologies, Via G. Salvatore, 486, 80145 Naples, Italy.
Scott D Sagel, Umer Khan, Sonya L Heltshe, John P Clancy, Drucy Borowitz, Daniel Gelfond, Scott H Donaldson, Antoinette Moran, Felix Ratjen, Jill M VanDalfsen, Steven M Rowe. Clinical Effectiveness of Lumacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del-CFTR. A Clinical Trial. Ann Am Thorac Soc 2021 Jan;18(1):75-83.doi: 10.1513/AnnalsATS.202002-144OC. [Pubmed]

Scott Segal
To evaluate the effectiveness of LUM/IVA in children (6 yr or more) and adults (more than 18 yr) in a postapproval setting. A total of 193 patients initiated LUM/IVA, and 85% completed the study through 1 year. Baseline mean percent-predicted forced expiratory volume in 1 second (ppFEV1) was 85 (standard deviation, 22.4) in this cohort. No statistically significant change in ppFEV1 was observed from baseline to any of the follow-up time points, with a mean absolute change at 12 months of -0.3 (95% confidence interval [CI], -1.8 to 1.2). Body mass index improved from baseline to 12 months (mean change, 0.8 kg/m2; P < 0.001). Sweat chloride decreased from baseline to 1 month (mean change, -18.5 mmol/L; 95% CI, -20.7 to -16.3; P < 0.001), and these reductions were sustained through the study period. There were no significant changes in hospitalization rate for pulmonary exacerbations and Pseudomonas aeruginosa infection status with treatment.
Conclusions: In this real-world multicentre cohort of children and adults, LUM/IVA treatment was associated with significant improvements in growth and reductions in sweat chloride without statistically significant or clinically meaningful changes in lung function, hospitalization rates, or P. aeruginosa infection. (NCT02477319).
Dr Scott D Sagel is Professor Pediatrics and Pulmonary Medicine at the Department of Pediatrics, Children’s Hospital Colorado and University of Colorado Anschutz Medical Campus, Aurora, Colorado.
Eva Furstova, Tereza Dousova, Jakub Beranek, Malgorzata Libik, Libor Fila, Martin Modrak, Ondrej Cinek, Milan Macek Jr, Pavel Drevinek Response to elexacaftor/tezacaftor/ivacaftor in intestinal organoids derived from people with cystic fibrosis. J Cyst Fibros 2021 Aug 1;S1569-1993(21)01304-7.doi: 10.1016/j.jcf.2021.07.006.Online ahead of print.[Pubmed]

Eva Furstova
Superior efficacy of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) over tezacaftor/ivacaftor (TEZ/IVA) in people with cystic fibrosis (CF) and Phe508del/Phe508del genotype was shown in clinical trials. We utilized intestinal organoid approach to compare in vitro responses to these 2 CFTR modulator drug combinations and to check potential inter-individual variability in therapeutic response to the triple combination.
Organoids from 17 subjects with Phe508del/Phe508del were screened with forskolin induced swelling assay. Significantly larger swelling, when exposed to ELX/TEZ/IVA as compared to TEZ/IVA, was observed in 16 of them. However, 1 sample showed no additional effect of ELX. The finding of unique CFTR variants in this sample indicates that genetic traits other than CF-causing CFTR mutation are worth exploring as they may have an impact on the definitive modulator drug response.
Dr Eva Furstova is a medical doctor and PhD researcher in the Department of Paediatrics, 2nd Faculty of Meicine, Charles University and Motol University Hospital, Prague, Czech Republic; Department of Medical Microbiology, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic
Donatello Salvatore, Carmela Colangelo, Michele D’Andria, Giovanni Marsicovetere, Domenica Passarella. Elexacaftor/tezacaftor/ivacaftor as rescue therapy in a patient with the cystic fibrosis genotype F508DEL/ G1244E. Clin Case Rep 2021 Aug 25;9(8):e04713.doi: 10.1002/ccr3.4713.eCollection 2021 Aug. OPEN access for full case reports [Pubmed]

Donatello Salvatore
Elexacaftor/tezacaftor/ivacaftor (ETI) is a cystic fibrosis (CF) transmembrane regulator (CFTR) modulator. It is known to be efficacious in stable patients with severe pneumopathy, but there are few data concerning its effectiveness during acute exacerbations. We here describe its use in a woman with CF and respiratory failure.
A 50-year old woman with severe CF who eventually went into respiratory failure. Our patient had an F/G genotype and had been previously treated with ivacaftor but, after an initial improvement, experienced rapid disease progression (as has been previously described in ivacaftor-treated adults with G mutations), possibly aggravated by Bcc colonization. During her subsequent hospitalization, she did not improve significantly until ETI was administered, and, as all her other treatments remained unchanged, this suggests it played a role in her recovery. Furthermore, her lung disease continued to improve after she was discharged: There was no recurrence of PEx, no need for antibiotics, and her lung function and ABG parameters improved.
Dr Donatello Salvatore is Director of the Cystic Fibrosis Centre – Hospital San Carlo Potenza Italy.
Oded Breuer, David Shoseyov, Shifra Koretz, Nadia Alyan, Joel Reiter, Malena Cohen-Cymberknoh, Isaiah Wexler, Eitan Kerem. Ethical dilemma: ELX/TEZ/IVA or Lung Transplantation in Cystic Fibrosis and End Stage Lung Disease? Chest 2021 Sep 7;S0012-3692(21)03846-0.doi: 10.1016/j.chest.2021.08.073.Online ahead of print. [Pubmed]

Oded Breuer
Cystic fibrosis is caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. Novel, highly effective, modulator therapies correcting and potentiating CFTR function are changing the course of the disease. We present an ethical dilemma involving an 11-year-old child with CF and end stage lung disease. Shortly after starting treatment with Elexacaftor-Tezacaftor-Ivacaftor, the family received notification that a matched donor lung had been allocated. Clinical decision-making in this case is challenging as definitive data to medically support one treatment option over the other is limited. A survey of CF center team members was conducted for the purpose of this manuscript. Ethical principles that may guide us in these situations are discussed.
Overall, results of the survey present a lack of agreement as to the best approach in this situation. Physicians, when compared to other team members, are more likely to provide a specific recommendation vs. to present the information and let the family decide (odds ratio (95% confidence interval)=4.0 (1.2-12.8), p=0.021). A shared decision-making model, stressing our moral obligation as clinicians to respect autonomy by appreciating family values while offering to participate in the decision-making process and ensuring non-maleficence, is presented.
In summary, CFTR modulators affect the outcomes of CF disease and influence clinical decision-making. Current lack of data on long-term outcomes, in young patients with CF receiving effective modulator therapy, should not preclude CF team participation in decision-making. Shared decision-making which is focused on respecting autonomy is our preferred approach in these situations.
Donatello Salvatore, Angela Pepe, Vincenzo Carnovale, Fabio Majo, Rita Padoan, Serena Quattrucci, Marco Salvatore, Domenica Taruscio, Annalisa Amato, Gianluca Ferrari, Giuseppe Campagna Elexacaftor/tezacaftor/ivacaftor for CFTR variants giving rise to diagnostic uncertainty: Personalised medicine or over-medicalisation? J Cyst Fibros 2021 Sep 25;S1569-1993(21)01416-8. doi: 10.1016/j.jcf.2021.09.011.Online ahead of print. [Pubmed]
In order to assess the real-world frequency of the three categories that may give rise to diagnostic uncertainty (mutations uncertainly interpreted as being pathogenic, mutations of unknown significance, and non CF-causing variants), we queried the Italian CF Registry (ICFR) and found that 113 (2.06%) of the 5,489 subjects registered and genotyped in 2018 had at least one such mutation: nine had four different mutations uncertainly interpreted as being pathogenic; three had two different mutations of unknown significance; and 101 had ten different non CF-causing variants.
These findings highlight an important question concerning the eligibility of subjects without a clearly diagnostic mutation for CFTR modulator treatment because the number of currently eligi- ble patients has greatly increased and the annual retail cost of ETI exceeds $300,000 per patient, which is much more expensive than the cost of other chronic CF treatments.
On the one hand, CF physicians often have to provide care for patients with rare CF genotypes in clinical practice, and so there is a need to test the potential efficacy of approved modulators in the treatment of rare CF-causing genotypes. On the other, although it is true that robust clinical trial data may be very difficult (if not impossible) to obtain, we wonder whether in vitro data alone are sufficient to justify the use of a very expensive treatment, especially in the case of patients with a diagnosis of CF based on genotypes containing non-CF-causing variants or variants whose clinical effect is doubtful or unknown.
The introduction of second tier CFTR-extended genetic analyses in new-born screening programmes without filtering for all known CF variants with full penetrance would inevitably increase the number of eligible subjects. A significant number of infants with so-called CF transmembrane conductance regulator-related metabolic syndrome (CRMS)/cystic fibrosis screen positive, inconclusive diagnosis (CFSPID) would be considered eligible for HEMT, and their parents would probably expect them to receive it only in the presence of mutations that were simply verifiable on a free- access website. However, the diagnosis of CF is and may remain inconclusive for a long time.
The wise use of economic resources is an important consideration when attempting to improve the access to treatment of patients who could really benefit from HEMT.
Dr Donatello Salvatore is at the Cystic Fibrosis Centre, San Carlo Hospital, Potenza, Italy; Italian Cystic Fibrosis Registry, Rome Italy.
Kevin J Scully, Peter Marchetti, Gregory S Sawicki, Ahmet Uluer, Manuela Cernadas, Rebecca E Cagnina, John C Kennedy, Melissa S Putman. The effect of elexacaftor/tezacaftor/ivacaftor (ETI) on glycemia in adults with cystic fibrosis. J Cyst Fibros 2021 Sep 13;S1569-1993(21)01377-1.doi: 10.1016/j.jcf.2021.09.001.Online ahead of print.[Pubmed]

Kevin Scully
The impact of elexacaftor-tezacaftor-ivacaftor (ETI) on glycemic control is currently unknown. Our objective was to investigate the effect of ETI initiation on glycemia in adults with CF using continuous glucose monitoring (CGM).
Methods: In this prospective observational study, 34 adults with CF and at least one F508del CFTR mutation wore CGM sensors for 14 days prior to starting ETI and again 3-12 months after ETI initiation. Hypoglycemia symptoms were queried at each visit, and most recent anthropometric measures and spirometry data were obtained by chart review.
Results: Twenty-three participants completed the study. Compared to baseline, average glucose (AG), standard deviation (SD), % time >200 mg/dL, and peak sensor glucose decreased with ETI treatment, and % time in target range 70-180 mg/dL increased. Improvements in glycemic parameters were most notable in individuals with CFRD. There was no significant change in CGM-measured or self-reported hypoglycemia before and after ETI initiation.
Conclusion: Initiation of ETI in adults with CF was associated with improvement CGM-derived measures of hyperglycemia and glycemic variability with no effect on hypoglycemia. The authors suggest further studies are needed to investigate underlying etiology of these changes and the long-term impact of ETI on glycemic control in patients with CF.
Dr Kevin J Scully is attending physician and clinical fellow in the Division of Endocrinology, Boston Children’s Hospital, 333 Longwood Avenue, 6th Floor, Boston, MA, 02115, United States; Harvard Medical School, Boston MA, United States.
Rosara Bass, Jefferson N Brownell, Virginia A Stallings. The Impact of Highly Effective CFTR Modulators on Growth and Nutrition Status. Nutrients 2021 Aug 24;13(9):2907. doi: 10.3390/nu13092907 free article [Pubmed]

Rosara Bass
Dysfunctional CFTR contributes to increased energy expenditure, exocrine pancreatic insufficiency causing impaired dietary macronutrient digestion and absorption, intestinal dysbiosis, and impaired bile acid homeostasis. Poor nutritional status as a result of these mechanisms is associated with decreased lung function, worse clinical outcomes, and ultimately, increased mortality. Nutritional interventions addressing these mechanisms, such as pancreatic enzyme-replacement therapy and enteral caloric supplementation, have improved nutritional status and, by association, clinical outcomes. In the last decade, the advent of medications targeting defective CFTR proteins has revolutionized the care of patients with CF by reducing the overall impact of CFTR dysfunction. Below, we summarize the effects of highly effective CFTR modulators on nutritional status overall as well as specific factors including bile acid metabolism, pancreatic function, energy expenditure, and intestinal dysbiosis. The authors suggest the future of CF nutrition care will require a paradigm shift away from focusing on methods addressing CFTR dysfunction such as excess calorie provision and toward an individualized, holistic approach in the context of specific mutations and CFTR-directed therapy.
Table 1 (from full paper available via PubMed) Summary of the effects of highly effective CFTR modulators on markers of growth, nutritional status and gastrointestinal health.
| Outcome | Ivacaftor | ELEX-TEZ-IVA |
| Weight-Z | Increased | Increased |
| Height-Z | Increased in youth | To be determined |
| BMI-Z | Increased | Increased |
| Fat Mass | Increased | To be determined |
| Fat-Free Mass | Unchanged to increased | To be determined |
| Bile Acid Metabolism | Increased FGF-19 and decreased C4 | To be determined |
| CF Liver Disease | To be determined, case reports of improved steatosis | To be determined |
| Exocrine Pancreatic Insufficiency | Increased fecal elastase at younger ages | To be determined |
| Bicarbonate Secretion | Decreased time to reach pH 5.5 | To be determined |
| Energy Expenditure | Decreased | To be determined |
| GI Microbiome | Changes in intestinal flora and decreased calprotectin | To be determined |
Dr Rosara Bass is attending physician with the Division of Gastroenterology, Hepatology and Nutrition at the Children’s Hospital of Philadelphia, 3401 Civic Center Blvd, Philadelphia, PA 19104, USA.
– This is an excellent comprehensive review of the general effects of CFTR modulators on wide variety of factors relevant to nutrition
Ruth H Keogh, Rebecca Cosgriff, Eleni-Rosalina Andrinopoulou, Keith G Brownlee, Siobhán B Carr, Karla Diaz-Ordaz, Emily Granger, Nicholas P Jewell, Alex Lewin, Clemence Leyrat, Daniela K Schlüter, Maarten van Smeden, Rhonda D Szczesniak, Gary J Connett. Projecting the impact of triple CFTR modulator therapy on intravenous antibiotic requirements in cystic fibrosis using patient registry data combined with treatment effects from randomised trials Thorax 2021 Sep 23;thoraxjnl-2020-216265.doi: 10.1136/thoraxjnl-2020-216265.Online ahead of print. [Pubmed]

Ruth Keogh
Background: Cystic fibrosis (CF) is a life-threatening genetic disease, affecting around 10 500 people in the UK. Precision medicines have been developed to treat specific CF-gene mutations. The newest, elexacaftor/tezacaftor/ivacaftor (ELEX/TEZ/IVA), has been found to be highly effective in randomised controlled trials (RCTs) and became available to a large proportion of UK CF patients in 2020. Understanding the potential health economic impacts of ELEX/TEZ/IVA is vital to planning service provision.
Methods: We combined observational UK CF Registry data with RCT results to project the impact of ELEX/TEZ/IVA on total days of intravenous (IV) antibiotic treatment at a population level. Registry data from 2015 to 2017 were used to develop prediction models for IV days over a 1-year period using several predictors, and to estimate 1-year population total IV days based on standards of care pre-ELEX/TEZ/IVA. We considered two approaches to imposing the impact of ELEX/TEZ/IVA on projected outcomes using effect estimates from RCTs: approach 1 based on effect estimates on FEV1% and approach 2 based on effect estimates on exacerbation rate.
Results: ELEX/TEZ/IVA is expected to result in significant reductions in population-level requirements for IV antibiotics of 16.1% (~17 800 days) using approach 1 and 43.6% (~39 500 days) using approach 2. The two approaches require different assumptions. Increased understanding of the mechanisms through which ELEX/TEZ/IVA acts on these outcomes would enable further refinements to our projections.
Conclusions: This work contributes to increased understanding of the changing healthcare needs of people with CF and illustrates how Registry data can be used in combination with RCT evidence to estimate population-level treatment impacts.
Professor Ruth H Keogh is at the Department of Medical Statistics, London School of Hygiene & Tropical Medicine, London, UK
Lauryn A Benninger, Cesar Trillo, Jorge Lascano. CFTR modulator use in post lung transplant recipients. J Heart Lung Transplant 2021 Aug 26;S1053-2498(21)02482-7.doi: 10.1016/j.healun.2021.08.009. Online ahead of print. [Pubmed]

Lauryn Benninger
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator therapy has previously been contraindicated in solid organ transplant recipients. This was due to lack of data and concern for interactions with immunosuppressive drug regimens. However, in post-lung transplant recipients, CFTR modulators may improve extrapulmonary manifestations of cystic fibrosis without impacting graft function or immunosuppressive drug levels. Herein, we present our single center experience with the use of elexacaftor/tezacaftor/ivacaftor, Trikafta, in adult post-lung transplant recipients.
Dr Lauryn A Benninger is a pulmonologist in the Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Florida College of Medicine, Gainesville, Florida
Edith T Zemanick, Jennifer L Taylor-Cousar, Jane Davies, Ronald L Gibson, Marcus A Mall, Edward F McKone; McNally P; Ramsey BW; Rayment JH; Rowe SM; Tullis E; Ahluwalia N; Chu C; Ho T; Moskowitz SM; Noel S; Tian S; Waltz D; Weinstock TG; Xuan F; Wainwright CE; McColley SA. A Phase 3 Open-Label Study of ELX/TEZ/IVA in Children 6 Through 11 Years of Age With CF and at Least One F508del Allele.Am J Respir Crit Care Med 2021 Mar 18. doi: 10.1164/rccm.202102-0509OC.Online ahead of print.[Pubmed]

Edith Zermanick
Rationale: Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was shown to be efficacious and safe in patients aged 12 years and older with cystic fibrosis and at least one F508del-CFTR allele, but has not been evaluated in children <12 years of age.
Objectives: To assess the safety, pharmacokinetics, and efficacy of ELX/TEZ/IVA in children 6 through 11 years of age with F508del-minimal function or F508del-F508del genotypes.
Methods: In this 24-week open-label Phase 3 study, children (N=66) weighing <30 kg received 50% of the ELX/TEZ/IVA adult daily dose (ELX 100 mg once daily, TEZ 50 mg once daily, and IVA 75 mg every 12 hours) while children weighing ≥30 kg received the full adult daily dose (ELX 200 mg once daily, TEZ 100 mg once daily, and IVA 150 mg every 12 hours).
Measurements and main results: The primary endpoint was safety and tolerability. The safety and pharmacokinetic profiles of ELX/TEZ/IVA were generally consistent with those observed in older patients. The most common reported adverse events (AEs) included cough, headache, and pyrexia; in most of the children who had AEs, these were mild or moderate in severity. Through Week 24, ELX/TEZ/IVA treatment improved the ppFEV1 (10.2 percentage points; 95% confidence interval [CI], 7.9 to 12.6), Cystic Fibrosis Questionnaire-Revised respiratory domain score (7.0 points; 95% CI, 4.7 to 9.2), lung clearance index2.5 (-1.71 units; 95% CI, -2.11 to -1.30), and sweat chloride (-60.9 mmol/L; 95% CI, -63.7 to -58.2); body mass index-for-age z-score increased over the 24-week treatment period compared to the pre-treatment baseline.
Conclusions: Our results show ELX/TEZ/IVA is safe and efficacious in children 6 through 11 years of age with at least one F508del-CFTR allele, supporting its use in this patient population.
Dr Edith T Zemanick is Associate Professor at the Colorado Anschutz Medical Campus and Children’s Hospital Colorado, Department of Pediatrics, Aurora, Colorado, United States
Peter J Barry, Marcus A Mall, Antonio Álvarez1, Carla Colombo, Karin M de Winter-de Groot, Isabelle Fajac et al. VX18-445-104 Study Group Collaborators, Triple Therapy for Cystic Fibrosis Phe508del-Gating and -Residual Function Genotypes. N Engl J Med 2021 Aug 26;385(9):815-825.doi: 10.1056/NEJMoa2100665. [Pubmed]

Peter J Barry
Background: Elexacaftor-tezacaftor-ivacaftor is a small-molecule cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimen shown to be efficacious in patients with at least one Phe508del allele, which indicates that this combination can modulate a single Phe508delallele. In patients whose other CFTR allele contains a gating or residual function mutation that is already effectively treated with previous CFTR modulators (ivacaftor or tezacaftor-ivacaftor), the potential for additional benefit from restoring Phe508del CFTR protein function is unclear.
Methods: We conducted a phase 3, double-blind, randomized, active-controlled trial involving patients 12 years of age or older with cystic fibrosis and Phe508del-gating or Phe508del-residual function genotypes. After a 4-week run-in period with ivacaftor or tezacaftor-ivacaftor, patients were randomly assigned to receive elexacaftor-tezacaftor-ivacaftor or active control for 8 weeks. The primary end point was the absolute change in the percentage of predicted forced expiratory volume in 1 second (FEV1) from baseline through week 8 in the elexacaftor-tezacaftor-ivacaftor group
Results: After the run-in period, 132 patients received elexacaftor-tezacaftor-ivacaftor and 126 received active control. Elexacaftor-tezacaftor-ivacaftor resulted in a percentage of predicted FEV1 that was higher by 3.7 percentage points (95% confidence interval [CI], 2.8 to 4.6) relative to baseline and higher by 3.5 percentage points (95% CI, 2.2 to 4.7) relative to active control and a sweat chloride concentration that was lower by 22.3 mmol per liter (95% CI, 20.2 to 24.5) relative to baseline and lower by 23.1 mmol per liter (95% CI, 20.1 to 26.1) relative to active control (P<0.001 for all comparisons). The change from baseline in the Cystic Fibrosis Questionnaire-Revised respiratory domain score (range, 0 to 100, with higher scores indicating better quality of life) with elexacaftor-tezacaftor-ivacaftor was 10.3 points (95% CI, 8.0 to 12.7) and with active control was 1.6 points (95% CI, -0.8 to 4.1). The incidence of adverse events was similar in the two groups; adverse events led to treatment discontinuation in one patient (elevated aminotransferase level) in the elexacaftor-tezacaftor-ivacaftor group and in two patients (anxiety or depression and pulmonary exacerbation) in the active control group.
Conclusions: Elexacaftor-tezacaftor-ivacaftor was efficacious and safe in patients with Phe508del-gating or Phe508del-residual function genotypes and conferred additional benefit relative to previous CFTR modulators. (Funded by Vertex Pharmaceuticals; VX18-445-104 ClinicalTrials.gov number, NCT04058353.).
Dr Peter J Barry is at the Manchester University NHS Foundation Trust, Manchester, United Kingdom.
2022
Jonathan Guo, Anna Garratt, Andrew Hill. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros 2022 Feb 3;S1569-1993(22)00031-5.doi: 10.1016/j.jcf.2022.01.009.Online ahead of print. [Pubmed]
Jonathan Guo
Background: Time has seen management for Cystic Fibrosis (CF) advance drastically, most recently in the development of the disease-modifying triple combination therapy ivacaftor/tezacaftor/elexacaftor. There is currently limited evidence regarding both the global epidemiology of CF and access to this transformative therapy – and therefore where needs are not being met. Therefore, this study aims to define gaps in access to CF treatment.
Methods: Patient data were extracted from established CF registries. Where these were not available, literature searches were conducted alongside an international survey of 51 CF experts to determine the diagnosed patient population. National CF prevalence estimates were combined with registry data on estimated population coverage, to extrapolate the total estimated number of undiagnosed patients. Estimates of ivacaftor/tezacaftor/elexacaftor treatment coverage were extracted from publicly available sales summaries and pricing data.
Results: 162,428 [144,606-186,620] people are estimated to be living with CF across 94 countries. Of these, an estimated 105,352 (65%) are diagnosed, with 19,516 (12%) receiving triple combination therapy. We estimated 57,076 patients with undiagnosed CF. Owing to a paucity of high-quality data, estimates of undiagnosed CF in low- and middle-income countries are highly uncertain. Patient registries were available in 45 countries, and used to identify 90% of the estimated diagnosed population.
Conclusions: A significant CF patient burden exists in countries where disease-modifying drugs are unavailable, and final figures are likely underestimates. This analysis shows the potential to improve rates of diagnosis and treatment for CF, so a higher percentage of patients receive the most effective triple combination treatment.
Dr Jonathan Guo is in the Faculty of Medicine, Imperial College London, United Kingdom
Clémence Martin, Martine Reynaud-Gaubert, Rebecca Hamidfar, Isabelle Durieu, Marlène Murris-Espin, Isabelle Danner-Boucher, Raphaël Chiron, Sylvie Leroy, Benoit Douvry, Dominique Grenet. Laurent Mely, Sophie Ramel, Sylvie Montcouquiol, LydieLemonnier, Espérie Burnet, Jean-Louis Paillasseur, Jennifer Da Silva, Pierre-Régis Burgel. Sustained effectiveness of elexacaftor-tezacaftor-ivacaftor in lung transplant candidates with cystic fibrosis. J Cyst Fibros. 2022 Feb 2;S1569-1993(22)00032-7.doi: 10.1016/j.jcf.2022.01.012.Online ahead of print [Pubmed]

Clemence Martin
Background: Elexacaftor-tezacaftor-ivacaftor induces rapid clinical improvement in patients with cystic fibrosis (CF) and advanced pulmonary disease, often leading to suspend the indication for lung transplantation. Yet no long-term data is available in lung transplant candidates.
Methods: Lung transplant candidates (defined as being waitlisted for lung transplantation or considered for listing within 3 months) who have initiated elexacaftor-tezacaftor-ivacaftor were identified in the French cohort of patients with CF and advanced pulmonary disease. Patients were prospectively followed to evaluate treatment safety and effectiveness from initiation to July 20th, 2021.
Results: Among the 331 patients with advanced CF pulmonary disease who initiated elexacaftor-tezacaftor-ivacaftor, 65 were lung transplant candidates (17 listed for transplantation, 48 considered for listing within 3 months). Median [IQR] follow-up time was 363 [329; 377] days. At the end of the follow-up period, two patients were transplanted five and 11 days following treatment initiation, two were listed for transplantation, and 61 no longer met transplantation criteria. Improvement in percent predicted forced expiratory volume in 1 s (ppFEV1) at one month was +13.4% (95% confidence interval, 10.3%-16.5%; P < 0.0001) and remained stable thereafter. Treatment burden decreased substantially, with an 86% decrease in the need for intravenous antibiotics, 59% for oxygen therapy and 62% for non-invasive ventilation.
Conclusion: In lung transplant candidates eligible for elexacaftor-tezacaftor-ivacaftor, the rapid improvement following initiation of treatment persisted over one year with a reduction in treatment burden and lung transplantation could be safely deferred in most patients.
Clémence Martin is at the Université de Paris, Institut Cochin, InsermU1016, Paris, France; Respiratory Medicine and Cystic Fibrosis National Reference Center, Cochin Hospital, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France; ERN-Lung CF network, Frankfurt, Germany.
Amanda L Stapleton, Adam J Kimple, Jennifer L Goralski, S Mehdi Nouraie, Barton F Branstetter, Amber D Shaffer, Joseph M Pilewski, Brent A Senior, Stella E Lee, Anna C Zemke. Elexacaftor-Tezacaftor- Ivacaftor improves sinonasal outcomes in cystic fibrosis. J Cyst Fibros 2022 Mar 14;S1569-1993(22)00051-0.doi: 10.1016/j.jcf.2022.03.002.Online ahead of print. Free article [Pubmed]

Amanda Stapleton
Background: Many individuals with cystic fibrosis (CF) have chronic rhinosinusitis resulting in nasal obstruction, sinus infections, and repeated surgeries. Elexacaftor-tezacaftor-ivacaftor is a highly effective modulator therapy approved for individuals aged 6 years or older with CF who have at least one F508del allele or other responsive mutation. The current study tests the hypothesis that ELX/TEZ/IVA improves sinonasal disease in CF.
Methods: The study was a pre/post, observational cohort study conducted at two sites. Participants underwent a study visit prior to starting ELX/TEZ/IVA and a second visit at a median of 9 months on therapy. Each visit included sinus CT scan, rigid nasal endoscopy, and sweat chloride measurement. Symptoms were measured with the 22 item Sinonasal Outcome Test at scheduled intervals during the study. Regression models were used to test for improvement in symptoms, endoscopy, and CT scales.
Results: The study enrolled 34 individuals, with a median age of 27 years (range 12-60). Symptoms improved within 7 days of therapy and plateaued by day 28. Endoscopic crusting resolved and nasal polyposis improved, with a decrease in size or resolution of polyps. Sinus opacification and mucosal thickening improved on CT radiographs with treatment.
Conclusions: Sinonasal symptoms improved rapidly and durably for at least 180 days on ELX/TEZ/IVA therapy. Objective measures of disease including endoscopic and CT findings improved with ELX/TEZ/IVA.
Dr Amanda L Stapleton is Associate Professor in the Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh, United States.
Thomas Simon FitzMaurice, Dilip Nazareth, Kapil Iyer, Martin Walshaw, Mohamed Al-Aloul. Elexacaftor/Tezacaftor/Ivacaftor as a Bridge to Lung Retransplant in a Recipient With Cystic Fibrosis. Exp Clin Transplant 2022 Mar 15.doi: 10.6002/ect.2021.0468.Online ahead of print. Free article [Pubmed]
The triple-combination cystic fibrosis transmembrane conductance regulator modulator elexacaftor/- tezacaftor/ivacaftor is known to improve lung function and have extrapulmonary benefits in people with cystic fibrosis. However, there is limited evidence for its use in patients with cystic fibrosis after lung transplant, where the donor lung expresses normal levels of the cystic fibrosis transmembrane conductance regulator. We describe the use of elexacaftor/tezacaftor/ivacaftor as a bridge to potential lung retransplant in a 37-year-old man with cystic fibrosis and chronic lung allograft dysfunction. Although forced expiratory volume in 1 second did not improve, the patient had decreased sputum volume, no pulmonary exacerbations of cystic fibrosis, and no longer required continuous antibiotic therapy. Pancreatic function, revised Cystic Fibrosis Questionnaire scores, sinus symptoms, weight, and corticosteroid dependence significantly improved. There were no reported side effects attributable to elexacaftor/tezacaftor/ivacaftor. However, the patient exhibited declined renal function, which had been initially attributed to lability in cyclosporin levels but which were corrected after lithotripsy for renal calculi.
Triple-combination modulators of the cystic fibrosis transmembrane conductance regulator may offer benefits to carefully selected individuals awaiting retransplant, balanced against the risk of worsened immunosuppressant level control.
Thomas Simon FitzMaurice is a Fellow in the Adult CF Unit, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom and the Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom.
Marco Zampoli, Nataliya Kashirskaya, Bulent Karadag, Luiz Vicente Ribeiro Ferreira da Silva Filho, Grace R Paul, Christine Noke . Global access to affordable CFTR modulator drugs: Time for action! J Cyst Fibros 2022 Mar 24;S1569-1993(22)00083-2.doi: 10.1016/j.jcf.2022.03.006.Online ahead of print.[Pubmed]
There is no abstract so the article is reproduced here in view of its important content.

Marco Zampoli
“Whilst the incredible advancements in CF treatment are to be applauded and celebrated, the reality is that only a minority of people with CF in the rich global North are benefiting from CFTRm therapy. We are therefore very grateful and encouraged by the timely and important article by Jonathan Guo et al. published recently in the Journal which highlights the stark reality and disparity that exists in CF diagnosis and treatment across the world. According to their estimates based on available data, there are 57,076 people with undiagnosed CF across the world with the highest burden likely to be in the Middle East and India. Furthermore, only 12% of estimated 105,352 people captured in global CF registries are receiving Trikafta®/Kaftrio® and the majority of them are living in North America or Western Europe. We agree with their view that paucity of epidemiological data and lack of CF diagnosis capacity in low and middle-income countries (LMICs) are major barriers to advocating for effective and equitable treatment in these regions. However, there are significant CF populations living in LMICs such as South Africa, India, the Middle East, eastern Europe, and South America who currently have no access nor pathway to registration of Trikafta®/Kaftrio® which will undoubtably lead to increasing disparity in CF outcomes between the rich global North and poor global South. In these countries, the main barrier to accessing triple CFTRm therapy is not lack of diagnosis capacity, but profit-driven global market forces which are accountable more to shareholders than the health needs of the global CF community.
Global distribution and sales of CFTRm drugs and patents are in place until 2037 in many LMICs that are signatories to the World Trade Organization Agreement on Trade-Related Aspects of Intellectual Property Rights, effectively preventing manufacture and distribution of affordable generic alternatives in these countries. The current annual cost per patient of Trikafta®/Kaftrio® paid by countries with negotiated agreements is more than $250 000 which is prohibitive for governments and private health insurers in LMICs. Vertex Pharmaceuticals posted a net income of $5.7 billion in 2021 which is a bitter pill to swallow for CF communities in LMICs who are helpless and increasingly impatient with the injustice of their plight . Precedent exists where pharmaceutical companies holding patents have issued voluntary licenses to generic manufacturers to distribute, with profit, life-saving affordable generic drugs in LMICs for treatment of Human Immunodeficiency Virus, hepatitis-C and more recently COVID-19. It is time that the global CF community stand in solidarity in support of similar issuing of voluntary licenses for the manufacture and distribution of generic CFTRm drugs.
We appeal and urge that the Journal and CF patient organizations in the global North such as the Cystic Fibrosis Foundation and European CF Society adopt a stronger position and publish statements against the injustice of the disparity in access to CFTRm therapy for all people across the world living with CF who are eligible for this life-saving treatment. Cystic fibrosis clinicians, researchers and CF communities living in LMICs cannot endure and tolerate much longer reading and hearing about the ‘miracle’ outcomes of triple CFTRm drugs of the privileged minority in rich countries. In the meantime, we continue to patiently negotiate in good faith with Vertex Pharmaceuticals in the hope that CF communities in LMICs will soon have affordable access to this life saving treatment. But time is running out for many people slowly dying too early with CF.
Dr Marco Zampoli is Clinical Head: Cystic Fibrosis Clinic Red Cross War Memorial Children’s Hospital, National Director South African CF Registry Steering Committee, Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Eunjin Hong, Lisa M Almond, P S Chung, A Peter Rao, Paul M Beringer PBPK-led guidance for cystic fibrosis patients taking elexacaftor-tezacaftor-ivacaftor with nirmatrelvir-ritonavir for the treatment of COVID-19. Clin Pharmacol Ther 2022 Mar 16.doi: 10.1002/cpt.2585. Online ahead of print. [Pubmed]

Eunjin Hong
Cystic fibrosis transmembrane conductance regulator (CFTR) modulating therapies including elexacaftor, tezacaftor, and ivacaftor (ETI) are primarily eliminated through cytochrome P450 (CYP) 3A-mediated metabolism. This creates a therapeutic challenge to the treatment of COVID-19 with nirmatrelvir-ritonavir in people with cystic fibrosis (CF) due to the potential for significant drug-drug interactions (DDI). However, CF population is more at risk of serious illness following COVID-19 infection and hence it is important to manage the DDI risk and provide treatment options.
CYP3A-mediated DDI of ETI was evaluated using a physiologically based pharmacokinetic (PBPK) modeling approach. Modeling was performed incorporating physiological information and drug dependent parameters of ETI to predict the effect of ritonavir (the CYP3A inhibiting component of the combination) on pharmacokinetics of ETI. The ETI models were verified using independent clinical pharmacokinetic and DDI data of ETI with a range of CYP3A modulators. When ritonavir was administered on day 1 through 5, the predicted AUC ratio of ivacaftor (the most sensitive CYP3A substrate) on day 6 was 9.31, indicating that its metabolism was strongly inhibited.
Based on the predicted DDI, the dose of ETI should be reduced when co-administered with nirmatrelvir-ritonavir to elexacaftor 200mg-tezacaftor 100mg-ivacaftor 150mg on days 1 and 5, with delayed resumption of full dose ETI on day 9, considering the residual inhibitory effect of ritonavir as a mechanism-based inhibitor. The simulation predicts a regimen of ETI administered concomitantly with nirmatrelvir/ritonavir in people with CF that will likely decrease the impact of the drug interaction.
Eunjin Hong is a graduate student the Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, 1985 Zonal Ave, Los Angeles, CA, 90033, USA.
Claire E Wainwright A New Era for Cystic Fibrosis and CFTR Modulator Trials in Infants. Am J Respir Crit Care Med 2022 Jul 20.doi: 10.1164/rccm.202207-1356ED.Online ahead of print.[Pubmed]
(Summary as no abstract)

Claire Wainwright
As CFTR modulator therapies becomes established in young children a new approach to clinical care is likely to evolve as people with CF live longer lives ensuring healthy ageing will become a priority
In open label trials nearly 11% of children had transaminases elevation more than 3x upper limit of normal. Details of trails in young infants remain to be decided – open label trials appear to be extending to infants. There may be unrecognised effects on the young developing child and will open label trials be adequate to recognise these problems?
As CFTR modulation becomes established clinically the window for randomised placebo-controlled trials has almost closed. Consumers, clinicians and researchers need to rapidly determine how we move forward in developing new CFTR modulator therapy for infants and decide whether infants are indeed “little adults” and whether benefits can be effectively inferred or whether they deserve the same degree of evidence as older people expect?
Claire E Wainwright is Professor of Paediatrics and Child Health University of Queensland, Brisbane, Australia
Brittany Miles, Jay Chacko, Mohammed Zaidan. The Impact of Elexacaftor/Ivacaftor/Tezacaftor on Cystic Fibrosis Patients Who Acquire COVID-19 Infection Cureus. 2022 Sep 17;14(9):e29276. doi: 10.7759/cureus.29276. eCollection 2022 Sep pubmed.ncbi.nlm.nih.gov/36277555
The combination of medication containing elexacaftor, ivacaftor, and tezacaftor (EIT) has dramatically impacted the treatment and prognosis for patients with cystic fibrosis (CF). Lung function, weight, and self-reported quality of life have improved for many of these patients, but little is known about whether this treatment will have a beneficial effect in preventing morbidity and/or mortality from respiratory infections such as COVID-19. EIT received Food and Drug Administration (FDA) approval shortly before the first cases of COVID-19 appeared in the United States. We performed an analysis using the TriNetX (Cambridge, MA, USA) research database to determine if patients being treated with EIT who became infected with COVID-19 experienced significantly different outcomes compared to patients who were not receiving it.
Brittany Miles is at Medical Education and Radiology, University of Texas Medical Branch, Galveston, USA.
Meliksah Arslan, Sarah Chalmers, Kelly Rentfrow Janelle M Olson , Vicki Dean , Mark E Wylam , Nadir Demirel. Suicide attempts in adolescents with cystic fibrosis on Elexacaftor/Tezacaftor/Ivacaftor therapy. Case Reports J Cyst Fibros
. 2023 Feb 7;S1569-1993(23)00023-1. doi: 10.1016/j.jcf.2023.01.015. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36759252/
Elexacaftor/Tezacaftor/Ivacaftor (ETI) is a recently approved cystic fibrosis (CF) transmembrane conductance regulator modulator therapy that has shown promising clinical and laboratory improvements on multiple organ systems in people with CF (pwCF). While original clinical trials found little to no effect on depression and anxiety, many post-marketing reports have suggested that ETI may be associated with adverse mental health effects. Here we report on two pwCF with adverse mental health effects shortly after starting ETI. Although many factors such as the burden of living with a chronic disease or widespread effects of the Covid-19 pandemic may have contributed to these events, similar reports have led to mounting concern that ETI may be the cause of such events. Regular mental health screening before the initiation of ETI and monitoring for signs and symptoms of mental diseases afterward should be a routine part of care, given the gravity of possible outcomes.
Meliksah Arslan is at theMarmara University School of Medicine, Istanbul, Turkey.
Christina J Bathgate, Emily Muther , Anna M Georgiopoulos, Beth Smith 4, Laura Tillman, Sonia Graziano, Marieke Verkleij, Paula Lomas 8, Alexandra Quittner. Positive and negative impacts of elexacaftor/tezacaftor/ivacaftor: Healthcare providers’ observations across US centers.Pediatr Pulmonol
. 2023 Jun 2. doi: 10.1002/ppul.26527. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37265418/

Christina Bathgate
Background: Elexacaftor/tezacaftor/ivacaftor (ETI) has been associated with unprecedented clinical improvements, transforming the management of cystic fibrosis (CF). However, side effects with implications for safety and well-being have been reported, including neuropsychiatric changes. This study aimed to better characterize the emerging positive and negative impacts of ETI.
Methods: The Cystic Fibrosis Foundation’s Mental Health Advisory Committee distributed a 26-item survey to US CF care teams to assess clinician observations of patient-reported experiences with ETI. Survey responses measured the prevalence of these effects in five domains: (1) positive physical and psychological effects, (2) sleep difficulties, (3) cognitive difficulties, (4) worsening mental health, and (5) concerns about the future and finances.
Results: Seventy-five healthcare providers responded from a pediatric, adult, and combined centers. Positive physical effects of ETI and increased optimism were reported in the upper quartiles (50%-100%) and rated as having a significant impact on daily functioning. Sleep and cognitive difficulties were reported in 1%-24%, with slight impacts on functioning, and psychological symptoms (e.g., increased stress, depression, anxiety) and new psychiatric medications were reported in 1%-24%, with moderate impacts. Concerns about the future were reported in 1%-24%, with minimal impacts.
Conclusion: Across US centers, providers most often observed positive physical effects of ETI. However, a variety of negative side effects were also reported, including sleep disruptions and worsening psychological functioning, which should be systematically monitored by CF teams. These national-level data are a first step in evaluating the prevalence and consequences of these side effects and can directly inform future studies
Liron Birimberg-Schwartz , Wan Ip , Claire Bartlett , Julie Avolio , Jeffrey M Beekman , Johanna Rommens, Lisa Strug , Christine E Bear , Theo J Moraes , Tanja Gonska. Validating organoid-derived human intestinal monolayers for personalized therapy in cystic fibrosis.Life Sci Alliance
. 2023 Apr 5;6(6):e202201857. doi: 10.26508/lsa.202201857. Print 2023 Jun. 37024122 Free PMC article pubmed.ncbi.nlm.nih.gov/37024122/

Liron Birimberg-Schwartz
gastro.on.ca
Highly effective drugs modulating the defective protein encoded by the CFTR gene have revolutionized cystic fibrosis (CF) therapy. Preclinical drug-testing on human nasal epithelial (HNE) cell cultures and 3-dimensional human intestinal organoids (3D HIO) are used to address patient-specific variation in drug response and to optimize individual treatment for people with CF.
This study is the first to report comparable CFTR functional responses to CFTR modulator treatment among patients with different classes of CFTR gene variants using the three methods of 2D HIO, 3D HIO, and HNE. Furthermore, 2D HIO showed good correlation to clinical outcome markers. A larger measurable CFTR functional range and access to the apical membrane were identified as advantages of 2D HIO over HNE and 3D HIO, respectively. Our study thus expands the utility of 2D intestinal monolayers as a preclinical drug testing tool for CF.
Liron Birimberg-Schwartz is in Department of Paediatrics, Division of Gastroenterology, Hepatology and Nutrition, University of Toronto, Toronto, Canada.and Translational Medicine, The Hospital for Sick Children, Toronto, Canada.
— The subject is reviewed in detail in the Free PMC article
Julie K Bower, Nataliya Volkova, Neil Ahluwalia, Gurvaneet Sahota, Fengjuan Xuan, Anna Chin, Tanya G Weinstock, Josh Ostrenga, Alexander Elbert. Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: Interim results of a long-term registry-based study. J Cyst Fibros
. 2023 Mar 22;S1569-1993(23)00066-8. doi: 10.1016/j.jcf.2023.03.002. Online ahead of print. Free article. pubmed.ncbi.nlm.nih.gov/36963986/

Julie K Bower
Vertex
Background: Phase 3 clinical trials showed elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was safe and efficacious in people with cystic fibrosis (CF) with ≥1 F508del-CFTR allele. To assess long-term effects of ELX/TEZ/IVA under real-world conditions of use, a 5-year observational registry-based study is being conducted. We report interim results from the first 2 years of follow-up.
Methods: The study included people with CF in the US Cystic Fibrosis Foundation Patient Registry (CFFPR) who initiated ELX/TEZ/IVA between October 2019 and December 2020. Pulmonary exacerbations (PEx), percent predicted forced expiratory volume in 1 second (ppFEV1), hospitalizations, bacterial pathogens, body mass index (BMI), CF complications and comorbidities, and liver function tests (LFTs) after treatment initiation were compared with the 5-year pre-treatment period. Death and lung transplantation were assessed relative to 2019 CFFPR data.
Results: 16,116 people with CF were included (mean treatment duration 20.4 months). Among those with 5 years of pre-treatment data, mean PEx/patient/year declined to 0.18 (95% CI: 0.17, 0.19) in Years 1 and 2 post-treatment from 0.86 (95% CI: 0.83, 0.88) in the baseline year (79% reduction), after a continued increase observed pre-treatment. Similarly, a decline in mean hospitalizations/patient/year was observed in Year 1 that was sustained in Year 2 (74% reduction from baseline year). The mean absolute change in ppFEV1 from baseline was +8.2 percentage points (95% CI: 8.0, 8.4) in Year 1 and +8.9 percentage points (95% CI: 8.7, 9.1) in Year 2, after a continued decline observed pre-treatment. Positive bacterial cultures decreased for all evaluated pathogens, and mean BMI increased by 1.6 kg/m2 (95% CI: 1.5, 1.6) by Year 2. No new safety concerns were identified based on evaluation of CF complications, comorbidities, and LFTs. The annualized rates of death (0.47% [95% CI: 0.39, 0.55]) and lung transplantation (0.16% [95% CI: 0.12, 0.22]) were considerably lower than reported in 2019 (1.65% and 1.08%, respectively).
Conclusions: ELX/TEZ/IVA treatment was associated with sustained improvements in lung function, reduced frequency of PEx and all-cause hospitalization, increased BMI, and lower prevalence of positive bacterial cultures. Additionally, there was a 72% lower rate of death and 85% lower rate of lung transplantation relative to the year before ELX/TEZ/IVA availability. These results, from the largest cohort of ELX/TEZ/IVA-treated people to date, extend our understanding of the broad clinical benefits of ELX/TEZ/IVA.
Julie K Bower is Associate Director of Clinical Epidemiology, Global Patient Safety at Vertex Pharmaceuticals, Boston, MA, United States of America.
Giuseppe Cimino , Sara Sorrenti , Manuel Murciano, Paola Galoppi, Fiorentina Ascenzioni, Bruno Botta, Roberto Brunelli ; Sapienza University Working Group on Cystic Fibrosis in Pregnancy. Use of elexacaftor/tezacaftor/ivacaftor combination in pregnancy. Review Arch Gynecol Obstet. 2023 Mar 13. doi: 10.1007/s00404-023-06962-5. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36907900/

Giuseppe Cimino obbervatoriomalattierare
Introduction: Management of cystic fibrosis has recently stepped forward with the introduction of cystic fibrosis transmembrane conductance regulator (CFTR) modulators, although data on potential adverse effects are lacking for many categories of patients, such as pregnant women.
Methods: We report one of the first reports on the outcome of pregnancy in a woman treated with Elexacaftor/Tezacaftor/Ivacaftor during the second and third trimester of pregnancy, showing a significant improvement of respiratory status, compared with the first trimester when the medication was discontinued due to unknown and, therefore, potential teratogenic effects. Also, we performed the review of the existing literature on the topic
Results: The course of pregnancy was uneventful, with reference to major obstetric complications, and the patient delivered a healthy neonate. These results were similar to those coming from other short series of pregnant women affected by cystic fibrosis and treated with CFTR modulators during pregnancy.
Conclusions: Thus, despite the lack of evidence on the topic, the use of Elexacaftor/Tezacaftor/Ivacaftor in pregnancy seems to be apparently not associated with major adverse events, thus opening optimistic scenarios in terms of management of these patients.
Dr Giuseppe Cimino is at the Fibrosis Regional Reference Center, A.O.U. Policlinico Umberto I, Rome, Italy. and the Department of Maternal and Child Health and Urological Sciences, Sapienza University of Rome, Via del Policlinico, 155, 00161, Rome, Italy.
Stefanie Dillenhoefer , Dorothy Grogono , Ana Morales-Tirado.A year in review (2022): Modulators and COVID19, the story goes on….. Review J Cyst Fibros. 2023 Mar 6;S1569-1993(23)00065-6. doi: 10.1016/j.jcf.2023.03.001. Online ahead of print. Free PMC article pubmed.ncbi.nlm.nih.gov/36906393/

Stefanie Dillenhoefer Linkedin
In this review the authors state they have aimed to cover some of the highlights of the cystic fibrosis (CF) literature in 2022, which again has been dominated by the SARS-CoV-2 (COVID-19) pandemic and modulator therapy. They have also explored other themes, such as novel findings in relation to pulmonary exacerbations, early lung disease and emerging areas for people with CF (pwCF).
– The free PMC article, accessed through the above PubMed link, is excellent and covers the main advances during the year.
Dr Stefanie Dillenhoefer is in the Department of Pediatric Pulmonology, Cystic Fibrosis Center, University Children’s Hospital of Ruhr University Bochum at St. Josef-Hospital, 44791 Bochum, Germany.
J Stuart Elborn , Francesco Blasi , Pierre-Régis Burgel , Daniel Peckham. Role of inhaled antibiotics in the era of highly effective CFTR modulators. Review Eur Respir Rev. 2023 Jan 11;32(167):220154. doi: 10.1183/16000617.0154-2022. Print 2023 Mar 31. Free article pubmed.ncbi.nlm.nih.gov/36631132/

Stuart Elborn
Recurrent and chronic bacterial infections are common in people with cystic fibrosis (CF) and contribute to lung function decline. Antibiotics are the mainstay in the treatment of exacerbations and chronic bacterial infection in CF. Inhaled antibiotics are effective in treating chronic respiratory bacterial infections and eradicating Pseudomonas aeruginosa from the respiratory tract, with limited systemic adverse effects. In the past decade, highly effective cystic fibrosis transmembrane conductance regulator (CFTR) modulators have become a new therapy that partially corrects/opens chloride transport in patients with selected CFTR mutations, restoring mucus hydration and improving mucociliary clearance. The recent triple CFTR modulator combination is approved for ∼80-90% of the CF population and significantly reduces pulmonary exacerbations and improves respiratory symptoms and lung function. CFTR modulators have shifted the focus from symptomatic treatment to personalised/precision medicine by targeting genotype-specific CFTR defects. While these are highly effective, they do not fully normalise lung physiology, stop inflammation or resolve chronic lung damage, such as bronchiectasis. The impact of these new drugs on lung health is likely to change the future management of chronic pulmonary infections in people with CF. This article reviews the role of inhaled antibiotics in the era of CFTR modulators.
Prof. Stuart Elbornis in the Faculty of Medicine Health and Life Sciences, Queen’s University, Belfast, UK s.elborn@qub.ac.uk.
Danielle M Goetz, Carla K Frederick , Geovanny Perez , Drucy Borowit Airway clearance after highly effective CFTR modulators: Normalizing life and reducing treatment burden. Pediatr Pulmonol. 2023 May 26. doi: 10.1002/ppul.26502. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37232336/

Danielle Goetz
Objectives: Airway clearance therapy (ACT) is an important component of therapy for cystic fibrosis (CF) but is associated with significant treatment burden. Highly effective CFTR modulator therapy (HEMT) has improved pulmonary function for many people with CF (pwCF). We sought to understand changes in attitudes and practices about ACT in the post-HEMT era.
Study design: Surveys of CF community members and CF care team members.
Methodology: Separate surveys were created for the CF community and CF care providers to evaluate attitudes towards ACT and exercise in the post-HEMT era. We solicited answers from pwCF via the CF Foundation’s Community Voice and from CF care providers via CF Foundation listservs. Surveys were available between July 20 and August 3, 2021.
Results: Surveys were completed by 153 community members (parents of children and pwCF) and 192 CF care providers. Belief that exercise can substitute partially for ACT was endorsed similarly by community members (59%) and providers (68%). After starting HEMT, 36% of parents of children and 51% of adults did fewer ACT treatments including 13% who stopped ACT. Adults reported altering their ACT regimen more than parents of children, though the sample size was limited. Half of providers had changed their ACT recommendations for those on HEMT. Fifty-three percent of respondents had discussed changing ACT with their care team (36% of parents, 58% of pwCF).
Conclusions: Providers should be aware that ACT management changes may have been undertaken by pwCF who have pulmonary benefits of HEMT. Treatment burden should be considered in co-management decisions regarding ACT and exercise.
Danielle M Goetz is Clinical Associate Professor in the Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, New York, USA.
Enery Gómez-Montes, Enrique Salcedo Lobato, Alberto Galindo, Diana García Alcázar, Cecilia Villalain, Maria Teresa Moral-Pumarega, Gerardo Bustos Lozano, Carmen Luna-Paredes. Prenatal cystic fibrosis transmembrane conductance regulator modulator therapy: A promising way to change the impact of cystic fibrosis. Case Reports Fetal Diagn Ther
. 2023 Mar 30. doi: 10.1159/000530261. Online ahead of print.pubmed.ncbi.nlm.nih.gov/36996799/

Enery Gomez-Montes Research Gate
Introduction: Cystic fibrosis (CF) is a potentially severe disease. The development of new therapies with cystic fibrosis transmembrane conductance regulator (CFTR) modulators has been a great advance in the management of this condition because they improve the function of the faulty CFTR protein rather than palliate its consequences. CFTR modulator therapy improves pancreatic and lung function and, therefore, quality of life, with greater benefits the sooner treatment is started. For this reason, the use of these therapies is being approved for increasingly younger patients. Only two cases of pregnant women taking CFTR modulators therapy with CF fetuses have been reported, suggesting that it could resolve meconium ileus (MI) prenatally, and delay/prevent other consequences of CF.
Case presentation: We report a case of a healthy pregnant patient who underwent CFTR modulator therapy with elexacaftor-tezacaftor-ivacaftor (ETI) in order to treat her fetus with CF (F508del homozygous CFTR mutation) and MI. Ultrasound findings suggestive of MI were observed at 24 weeks. Both parents were tested for CFTR mutations, and both were carriers of the F508del CFTR mutation. The fetus was diagnosed with CF by amniocentesis at 26+2 weeks. Maternal ETI therapy was initiated at 31+1 weeks and no dilated bowel was observed at 39 weeks. There were no signs of bowel obstruction after birth. Maternal ETI treatment was continued during breastfeeding, with normal liver function. Immunoreactive trypsinogen in the newborn was 58.1 ng/mL, sweat chloride test was 80 mmol/l, and fecal elastase on the second day of life was 58 μg/g.
Discussion/conclusion: Prenatal ETI treatment, as well as during breastfeeding, could solve, prevent and/or delay CF complications.
Every Gômez-Montes is at Fetal Medicine Unit, Department of Obstetrics and Gynecology, Research Institute Hospital 12 de Octubre (imas12), Primary Care Interventions to Prevent Maternal and Child Chronic Diseases of Perinatal and Developmental Origin (RICORS Network), University Hospital 12 de Octubre, Complutense University, Madrid, Spain
Jennifer L Goralski , Jordana E Hoppe , Marcus A Mall, Susanna A McColley , Edward McKone , Bonnie Ramsey , Jonathan H Rayment , Phil Robinson , Florian Stehling , Jennifer L Taylor-Cousar, Elizabeth Tullis , Neil Ahluwalia, Anna Chin , Chenghao Chu , Mengdi Lu, Tao Niu , Tanya Weinstock , Felix Ratjen , Margaret Rosenfeld ; VX20-445-111 Study Group.Phase 3 Open-Label Clinical Trial of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged 2 Through 5 Years with Cystic Fibrosis and at Least One F508del Allele. Am J Respir Crit Care Med. 2023 Mar 15. doi: 10.1164/rccm.202301-0084OC. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36921081/

Jennifer Goralski
UNC School of Medicine
Rationale: Elexacaftor/tezacaftor/ivacaftor has been shown to be safe and effective in people with cystic fibrosis (CF) aged ≥6 years with ≥1 F508del-CFTR allele but has not been studied in younger children.
Objectives: To evaluate the safety, pharmacokinetics, pharmacodynamics, and efficacy of elexacaftor/tezacaftor/ivacaftor in children with CF aged 2 through 5 years.
Methods: In this Phase 3, open-label, two-part study (Parts A and B), children weighing <14 kg (on Day 1) received elexacaftor 80 mg once daily, tezacaftor 40 mg once daily, and ivacaftor 60 mg each morning and 59.5 mg each evening; children weighing ≥14 kg received elexacaftor 100 mg once daily, tezacaftor 50 mg once daily, and ivacaftor 75 mg every 12 hours.
Measurements and main results: The primary endpoints for Part A (15-day treatment period) were pharmacokinetics and safety and tolerability. For Part B (24-week treatment period), the primary endpoint was safety and tolerability; secondary endpoints included pharmacokinetics and absolute changes from baseline in sweat chloride concentration and lung clearance index2.5 (LCI2.5) through Week 24. Analysis of pharmacokinetic data from 18 children enrolled in Part A confirmed the appropriateness of the Part B dosing regimen. In Part B, 75 children (F508del/minimal function genotypes, n = 52; F508del/F508del genotype, n = 23) were enrolled and dosed. Seventy-four children (98.7%) had adverse events which were all mild (62.7%) or moderate (36.0%) in severity. The most common adverse events were cough, fever, and rhinorrhea. Decreases in sweat chloride concentration (-57.9 mmol/L [95% confidence interval [CI], -61.3 to -54.6]; n = 69) and LCI2.5 (-0.83 units [95% CI, -1.01 to -0.66]; n = 50) were observed from baseline through Week 24. Mean BMI was within the normal range at baseline and remained stable at Week 24.
Conclusions: In this open label study in children 2 through 5 years of age, elexacaftor/tezacaftor/ivacaftor treatment was generally safe and well tolerated, with a safety profile consistent with that observed in older age groups, and led to clinically meaningful reductions in sweat chloride concentration and lung clearance index. Clinical trial registration available at www.
Dr Jennifer L Goralski is at the University of North Carolina at Chapel Hill, North Carolina, United States and is Associate Director of the Adult Cystic Fibrosis Center
Andrea Granados , Christine L Chan, Amir Moheet, Timothy Vigers, Ana María Arbeláez, Katie Larson Ode. The impact of elexacaftor/tezacaftor/ivacaftor on body composition in a small cohort of youth with cystic fibrosis. Pediatr Pulmonol. 2023 Mar 17. doi: 10.1002/ppul.26388. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36929859

Andrea Granado Docspot
Background: The effects of elexacaftor-tezacaftor-ivacaftor (ETI) on body composition in people with CF (pwCF) are unknown.
Methods: Dual-energy X-ray absorptiometry fat-free mass and fat mass adjusted for height (FMI) as well as oral glucose tolerance test derived measures of insulin secretion and sensitivity were compared before and after ETI initiation in eight pwC
Results: Patients median age: 22 years interquartile range (IQR: 16-28), 87.5% male, median time on ETI:11 months. Weight z-score increased from -0.52 to 0.18 (p = 0.014); FMI increased from 4.12 to 6.29 (p = 0.014). Insulin secretion (C pep iAUC/Gluc iAUC) increased from 8.71 to 14.21 (p = 0.021), insulin resistance (HOMA2 IR) increased from 0.73 to 1.25 (p = 0.014) and insulin sensitivity decreased (Matsuda) 8.88 to 5.58 (p = 0.036).
Conclusions: ETI resulted in increased weight and fat mass. BMI and muscle mass did not change. Both insulin secretion and insulin resistance increased. Longer-term metabolic consequences of ETI need further investigation.
Dr Andrea Granados is a pediatric endocrinologist in the Department of Pedaitrics, the Nicklaus Children’s Hospital, Miami, Florida USA
Emma L Guenther, Karen S McCoy , Mariah Eisner , Shasha Bai , Christopher J Nemastil , Kimberly J Novak, Terri Johnson, Emily M Stephan. Impact of chronic medication de-escalation in patients with cystic fibrosis taking elexacaftor, tezacaftor, ivacaftor: A retrospective review.J Cyst Fibros
. 2023 Apr 15;S1569-1993(23)00082-6. doi: 10.1016/j.jcf.2023.03.018. Online ahead of print.pubmed.ncbi.nlm.nih.gov/37069044/
Background: This single-center, retrospective study evaluated the effects of de-escalating cystic fibrosis (CF) supportive therapies in patients on elexacaftor/tezacaftor/ivacaftor (ETI). For many with CF, the clinical benefit of ETI exceeds that of supportive therapies. Therefore, we anticipated patients would desire to discontinue many of their supportive therapies, leading to the creation of a de-escalation algorithm. If patients were clinically improved and stable on ETI, CF supportive therapies could be de-escalated quarterly in accordance with the algorithm.
Methods: The primary objective was to assess non-inferiority of supportive therapies de-escalation by comparing the absolute change in percent predicted (ppFEV1) from baseline to month 1 versus the absolute change from baseline to month 12 after initiating ETI with patients serving as their own control. A chart review of patients initiated on ETI from September 2019 through December 2020 was conducted. Inclusion criteria included those six years and older with at least one copy of F508del.
Results: The study included 174 patients. The mean ppFEV1 at baseline, month 1, and month 12 was 67%, 78%, and 87% respectively. The mean difference in absolute change in ppFEV1 from baseline to month 1 compared to baseline to month 12 after the initiation of ETI was 1.53% (95% CI: -0.49 to 3.55)
CONCLUSION: De-escalating supportive therapies for those on ETI was non-inferior to remaining on all supportive therapies. This suggests that medications may be able to be discontinued under the context of a de-escalation algorithm, which may decrease medication burden and cost and increase quality of life.
Emma L Guenther is in the Department of Pharmacy, Nationwide Children’s Hospital, Columbus, OH, USA.
Steven Levitte, Yonathan Fuchs, Russell Wise , Zachary M Sellers. Effects of CFTR modulators on serum biomarkers of liver fibrosis in children with cystic fibrosis. J Hepatol Commun. 2023 Jan 20;7(2):e0010. doi: 10.1097/HC9.0000000000000010. Free article pubmed.ncbi.nlm.nih.gov/36662672/

Steven Levitte. LinkedIn
The cystic fibrosis (CF) transmembrane conductance regulator corrector/potentiator combinations lumacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor improve sweat chloride, pulmonary function, and nutrition. Yet it is unclear whether they may also impact the progression of liver fibrosis, which is a substantial source of morbidity and mortality for patients with CF. We conducted a retrospective, single-center analysis of children and adolescents with CF treated with lumacaftor/ivacaftor and/or elexacaftor/tezacaftor/ivacaftor therapy, focusing on alterations in liver function tests and fibrosis indices using previously-established thresholds that corresponded with increased liver elastography.
In pairwise comparisons of before and during treatment timepoints, we found that those with CF-associated liver involvement experienced significant decreases in gamma-glutamyl transferase, aspartate aminotransferase-to-platelet index, and gamma-glutamyl transferase-to-platelet ratio while on lumacaftor/ivacaftor. These differences were not observed in patients treated with elexacaftor/tezacaftor/ivacaftor, nor were they observed in patients without underlying CF-associated liver disease. These results provide the first evidence that lumacaftor/ivacaftor may improve liver fibrosis in children and adolescents with CF and suggest it may be beneficial in the treatment of CF-associated liver disease
Steven Levitt is in the Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Stanford University, Palo Alto, California, USA.
Karen S McCoy , Jill Blind , Terri Johnson, Patti Olson, Laura Raterman , Shasha Bai, Mariah Eisner, Shahid I Sheikh, Stephan Druhan, Cody Young, Kimberly Pasley. Clinical change 2 years from start of elexacaftor-tezacaftor-ivacaftor in severe cystic fibrosis .Pediatr Pulmonol 2023 Jan 17. doi: 10.1002/ppul.26318. Online ahead of print. hpubmed.ncbi.nlm.nih.gov/36650567/

Karen S McCoy. ResearchGate
Rationale: Limited published research is available on the impact of elexacaftor/tezacaftor/ivacaftor (ETI) beyond the initial few months postdrug initiation, especially for those who initiated therapy via individual investigational new drug application. The experiences of patients with cystic fibrosis (CF) experiencing severe lung disease were reviewed for significant improvements in clinical symptoms and quality of life.
Objectives: To examine clinical outcomes 2 years post-ETI in patients with CF and advanced lung disease.
Methods: This single center institutional review board-approved, retrospective chart review assessed clinical markers (percent predicted forced expiratory volume in 1 s, weight, sweat chloride), quality of life and computed tomography scans in patients with advanced lung disease who met criteria for compassionate use/expanded access program due to high risk of death or transplant need within 2 years.
Results: Eighteen identified patients (ages 15-49 years) initiated drug between July and September 2019. Clinical markers indicated that therapy was well tolerated, not discontinued by any participant, and lab values did not indicate medical concern or discontinuation. Monitoring results indicated the safety of modulator therapy as there were no adverse clinical occurrences and all patients presented universal stabilization. There were no deaths and no transplants by the end of the study.
Conclusions: This study focused on patients with CF eligible for modulator therapy and were initiated due to advanced lung disease. Initiation of modulator therapy was deemed safe and resulted in objective positive changes in nutrition, cough, FEV1 , subjective reports of clinical status, level of activity, and a reduction in burden of treatment.
Karen S McCoy is professor at the Pulmonary and Sleep Medicine Division, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Caoimhe McParland, Matthew Nunn, Theodore K Marras, Meredith Chiasson. Eradication of Mycobacterium abscessus infection in cystic fibrosis with initiation of Elexacaftor/Tezacaftor/Ivacaftor. Case Reports J Cyst Fibros
. 2023 Apr 17;S1569-1993(23)00095-4. doi: 10.1016/j.jcf.2023.03.021. Online ahead of print pubmed.ncbi.nlm.nih.gov/37076409/
Mycobacterium abscessus is a nontuberculous mycobacterium that is often multi-drug resistant, difficult to eradicate and associated with a rapid decline in lung function in cystic fibrosis (CF). Elexacaftor/Tezacaftor/Ivacaftor (ETI) is a combination CFTR modulator that improves lung function and decreases exacerbations, but limited data exists about its impact on respiratory infections. A 23-year-old male with CF (F508del, unknown) was diagnosed with Mycobacterium abscessus subspecies abscessus infection. He completed 12-weeks of intensive therapy, followed by oral continuation therapy. Antimicrobials were later discontinued for optic neuritis secondary to linezolid. He remained off antimicrobials with persistently positive sputum cultures. He then initiated ETI, and bronchoscopy eight months later suggested eradication of M. abscessus. By modulating CFTR protein function, ETI may improve innate airway defence mechanisms, facilitating the clearance of infections such as M. abscessus. This case highlights the potential positive implications of ETI on the challenging treatment of M. abscessus infections in CF.
Caoimhe McParland is in the Department of Medicine, Dalhousie University, Halifax, NS, Canada.
Margarete Olivier , Alexandra Kavvalou, Matthias Welsner, Raphael Hirtz, Svenja Straßburg, Sivagurunathan Sutharsan, Florian Stehling, Mathis Steindor. Real-life impact of highly effective CFTR modulator therapy in children with cystic fibrosis.Front Pharmacol
. 2023 May 9;14:1176815. doi: 10.3389/fphar.2023.1176815. eCollection 2023. pubmed.ncbi.nlm.nih.gov/37229253/

Margarete Olivier
Objective: To assess the intermediate term effects of elexacaftor/tezacaftor/ivacaftor in children with cystic fibrosis in a real-world setting. Methods: We performed a retrospective analysis of records of children with cystic fibrosis, who started elexacaftor/tezacaftor/ivacaftor between 8/2020 and 10/2022.
(Full details in PubMed link above)
Conclusion: In a real-world setting, elexacaftor/tezacaftor/ivacaftor therapy had beneficial clinical effects and a good safety profile in eligible children with cystic fibrosis comparable to previously published data from controlled clinical trials. The positive impact on pulmonary function tests and nutritional status seen after 3 months of elexacaftor/tezacaftor/ivacaftor therapy was sustained at 6 months follow-up.
Dr Margarete Olivier is at Pediatric Pulmonology and Sleep Medicine, Cystic Fibrosis Center, Children’s Hospital, University of Duisburg-Essen, Essen, Germany.
Nikita Padmakumar, Haji Sheeraz Khan. A foetus with cystic fibrosis – To treat or not to treat? Respir Med Res. 2023 Mar 5;83:101006. doi: 10.1016/j.resmer.2023.101006. Online ahead of print. Free article pubmed.ncbi.nlm.nih.gov/37037055/
Background: Cystic fibrosis (CF) is an autosomal recessive health condition caused by gene mutations causing quantitative and or qualitative defect in the cystic-fibrosis transmembrane conductance regulator (CFTR) protein. CFTR defects lead to abnormal ion transport affecting multiple body systems. In CF thick secretions accumulate causing impairment in the pancreas, whole airways, gut and reproductive organs.
Cftr modulators and pregnancy: CFTR modulators have improved the quantity and quality of life of CF patients. There is limited literature on CFTR modulator use in pregnancy and its impact on foetal health. A recent case report described a child with CF being born with pancreatic sufficiency following in-utero CFTR modulator exposure. We review the potential impact of in-utero exposure to CFTR modulators, focusing on pancreatic function and future fertility of unborn individuals with CF.
Conclusion: CFTR modulator exposure in-utero is a new concept, therefore the consequences on foetal health remain uncertain. Foetal exposure to modulators could prevent pancreatic damage and infertility.
Nikita Padmakumar is in the Department of Paediatrics, Hull University Teaching Hospitals, NHS Trust, Hull HU3 2JZ, UK.
— There is a useful review of the present information on the use of modulators during pregnancy.
Linus Piehler, Ralf Thalemann, Christine Lehmann, Stephanie Thee, Jobst Röhmel, Zulfiya Syunyaeva, Mirjam Stahl, Marcus A Mall, Simon Y GraeberEffects of elexacaftor/tezacaftor/ivacaftor therapy on mental health of patients with cystic fibrosis. Front Pharmacol. 2023 Apr 21;14:1179208. doi: 10.3389/fphar.2023.1179208. eCollection 2023. pubmed.ncbi.nlm.nih.gov/37153809/#full-view-affiliation-3
Introduction: The CFTR modulator drug elexacaftor/tezacaftor/ivacaftor (ETI) was shown to improve CFTR function and clinical symptoms in patients with cystic fibrosis (CF) with at least one F508del allele. Recently, some case reports suggested potential side effects of ETI on mental health with an increase in depressive symptoms and even suicide attempts in patients with CF. However, the general effects of this triple combination therapy on the mental health status of patients with CF remain largely unknown. Methods: We, therefore, performed a prospective, observational study in a real-life setting and investigated the relationship between initiation of ETI therapy and changes in mental health in adult patients with CF. We assessed Cystic Fibrosis Questionnaire-Revised (CFQ-R), Patient Health Questionnaire-9 (PHQ-9), Beck’s Depression Inventory – Fast Screen (BDI-FS) and Generalized Anxiety Disorder 7-item Scale (GAD-7) at baseline and 8-16 weeks after initiation of ETI. Results: In total, 70 adult patients with CF with at least one F508del allele and a median age of 27.9 years were recruited. After initiation of ETI, the CFQ-R respiratory domain score improved by 27.9 (IQR 5.6 to 47.2; p < 0.001). The PHQ-9 score of depressive symptoms decreased by 1.0 (IQR -3.0 to 0.3; p < 0.05) with an increase of 16.9% in the group with a minimal score after initiation of ETI and a decrease in the groups of mild (-11.3%) or moderate (-5.7%) scores compared to baseline. The BDI-FS score of depressive symptoms decreased from 1.0 (IQR 0.0-2.0) at baseline to 0.0 (IQR 0.0 to 2.0; p < 0.05) after initiation of ETI. The group with a minimal BDI-FS score increased by 8.0% after initiation of ETI, whereas the groups with mild (-4.9%), moderate (-1.6%) or severe (-1.6%) scores decreased compared to baseline. The GAD-7 score of anxiety symptoms did not change after initiation of ETI compared to baseline (0.0; IQR -2.0. to 0.0; p = 0.112). Conclusion: Initiation of ETI improves symptoms of depression in adult patients with CF with at least one F508del allele. However, symptoms of anxiety do not change after short-term therapy with ETI.
Linus Piehler is in the Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine and Cystic Fibrosis Center, Charité – Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
E de Poel, S Spelier, M C Hagemeijer, P van Mourik, S W F Suen, A M Vonk, J E Brunsveld, G N Ithakisiou, E Kruisselbrink, H Oppelaar , G Berker , K M de Winter de Groot , S Heida-Michel , S R Jans , H van Panhuis , M Bakker , R van der Meer , J Roukema, E Dompeling , E J M Weersink , G H Koppelman , A R Blaazer , J E Muijlwijk-Koezen , C K van der Ent, J M Beekman FDA-approved drug screening in patient-derived organoids demonstrates potential of drug repurpsing for rare cystic fibrosis genotypes. J Cyst Fibros
. 2023 May 3;S1569-1993(23)00067-X. doi: 10.1016/j.jcf.2023.03.004. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37147251/
Background: Preclinical cell-based assays that recapitulate human disease play an important role in drug repurposing. We previously developed a functional forskolin induced swelling (FIS) assay using patient-derived intestinal organoids (PDIOs), allowing functional characterization of CFTR, the gene mutated in people with cystic fibrosis (pwCF). CFTR function-increasing pharmacotherapies have revolutionized treatment for approximately 85% of people with CF who carry the most prevalent F508del-CFTR mutation, but a large unmet need remains to identify new treatments for all pwCF.
Methods: We used 76 PDIOs not homozygous for F508del-CFTR to test the efficacy of 1400 FDA-approved drugs on improving CFTR function, as measured in FIS assays. The most promising hits were verified in a secondary FIS screen. Based on the results of this secondary screen, we further investigated CFTR elevating function of PDE4 inhibitors and currently existing CFTR modulators.
Results: In the primary screen, 30 hits were characterized that elevated CFTR function. In the secondary validation screen, 19 hits were confirmed and categorized in three main drug families: CFTR modulators, PDE4 inhibitors and tyrosine kinase inhibitors. We show that PDE4 inhibitors are potent CFTR function inducers in PDIOs where residual CFTR function is either present, or created by additional compound exposure. Additionally, upon CFTR modulator treatment we show rescue of CF genotypes that are currently not eligible for this therapy.
Conclusion: This study exemplifies the feasibility of high-throughput compound screening using PDIOs. We show the potential of repurposing drugs for pwCF carrying non-F508del genotypes that are currently not eligible for therapies.
One-sentence summary: We screened 1400 FDA-approved drugs in CF patient-derived intestinal organoids using the previously established functional FIS assay, and show the potential of repurposing PDE4 inhibitors and CFTR modulators for rare CF genotypes.
Dr Ellen de Poel is in the Department of Pediatric Respiratory Medicine, Wilhelmina Children’s Hospital, University Medical Center, Utrecht University, Utrecht, EA 3584, the Netherlands; Regenerative Medicine Utrecht, University Medical Center, Utrecht University, Utrecht, CT 3584, the Netherlands.
Debanjali Purkayastha, Kyla Agtarap, Kristy Wong, Onella Pereira, Jannie Co, Smita Pakhale, Salmaan Kanji. Drug-drug interactions with CFTR modulator therapy in cystic fibrosis: Focus on Trikafta®/Kaftrio®. J Cyst Fibros. 2023 Jan 16;S1569-1993(23)00004-8. doi: 10.1016/j.jcf.2023.01.005. Online ahead of print.pubmed.ncbi.nlm.nih.gov/36653239/

Debanjali Purkayastha LinkedIn
The combination of CFTR modulators ivacaftor, tezacaftor and elexacaftor (Trikafta®, Kaftrio®) significantly improve outcomes, including survival in a broad range of cystic fibrosis patients. These drugs have complicated metabolic profiles that make the potential for drug interactions an important consideration for prescribers, care providers and patients. Prolonged survival also increases risk of age-related disease and their associated pharmacotherapy, further increasing the risk of drug interactions and the need for increased vigilance amongst care providers. We systematically searched the literature for studies identifying and evaluating pharmacokinetic and pharmacodynamic drug interactions involving the components of Trikafta®/Kaftrio®. We also searched electronic databases of drugs for possible drug interactions based on metabolic profiles. We identified 86 potential drug interactions of which 13 were supported by 14 studies. There is a significant need for research to describe the likelihood, magnitude and clinical impact of the drug interactions proposed here.
Debanjali Purkayastha is a Pharmacy Resident at the University of Waterloo, Kitchener, ON, Canada.
Ido Sadras , Malena Cohen-Cymberknoh , Eitan Kerem, Benjamin Z Koplewitz , Natalia Simanovsky, Michael Wilschanski, Liron Birimberg-Schwartz, Oded Breuer. Acute pancreatitis in pancreatic-insufficient cystic fibrosis patients treated with CFTR modulators. Case Reports J Cyst Fibros. 2023 Mar 11;S1569-1993(23)00064-4. doi: 10.1016/j.jcf.2023.02.013. Online ahead of print pubmed.ncbi.nlm.nih.gov/36914434/
Cystic fibrosis transmembrane conductance regulator modulator therapy is associated with substantial clinical benefit and improved quality of life in patients with cystic fibrosis (CF). While their effect on lung function has been clearly reported, we are still in the process of unraveling the full impact they have on the pancreas. We present two cases of pancreatic-insufficient CF patients who presented with acute pancreatitis shortly after commencing elexacaftor/tezacaftor/ivacaftor modulator therapy. Both patients were treated with ivacaftor for 5 years prior to elexacaftor/tezacaftor/ivacaftor initiation, but had no previous episodes of acute pancreatitis. We suggest that highly effective modulator combination therapy may restore additional pancreatic acinar activity, resulting in the development of acute pancreatitis in the interim until ductal flow is improved. This report adds to the growing evidence for possible restoration of pancreatic function in patients receiving modulator therapy, and highlights that treatment with elexacaftor/tezacaftor/ivacaftor may be associated with acute pancreatitis until ductal flow is restored, even in pancreatic-insufficient CF patients.
Ido Sadras is at the CF Center, Department of Pediatrics, Hadassah Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel.
L Schembri, S Warraich, S Bentley, S B Carr, I M Balfour-Lynn. Impact of elexacaftor/tezacaftor/ivacaftor on fat-soluble vitamin levels in children with cystic fibrosis. J Cyst Fibros 2023 May 2;S1569-1993(23)00125-X. doi: 10.1016/j.jcf.2023.04.019. Online ahead of print. https://pubmed.ncbi.nlm.nih.gov/37142523/

Laura Schembri
LinkedIn
Background: Children with cystic fibrosis are at risk of fat-soluble vitamin deficiency. CFTR modulators positively affect nutritional status. This study aimed to assess changes in serum vitamins A, D & E after starting ETI therapy to ensure levels were not abnormally high.
Methods: Retrospective review of annual assessment data over 3½ years, before and after starting ETI in a specialist paediatric CF centre, including vitamin levels.
Results: 54 eligible patients were included, aged 5-15 yrs (median age 11.5). Median time to post measurements was 171 days. Median vitamin A was increased (1.38 to 1.63 µmol/L, p<0.001). Three patients (6%) had high vitamin A post-ETI, compared with none at baseline; and 2 (4%) had low levels compared to 4 (8%) at baseline. No changes in vitamins D&E.
Conclusions: This study found increased vitamin A, sometimes to high levels. We recommend testing levels within 3 months of starting ETI
Laura Schembri is in the Department of Paediatric Dietetics, Royal Brompton Hospital, London, UK.
Mathias Schenkel , Dorna Ravamehr-Lake , Tomasz Czerniak , James P Saenz , Georg Krainer , Michael Schlierf , Charles M Deber. Impact of cholesterol and Lumacaftor on the folding of CFTR helical hairpins. Biochim Biophys Acta Biomembr. 2023 Jan 1;1865(1):184078. doi: 10.1016/j.bbamem.2022.184078. Epub 2022 Oct 22. pubmed.ncbi.nlm.nih.gov/36279907/

Mathias Schenkel
Cystic fibrosis (CF) is caused by mutations in the gene that codes for the chloride channel cystic fibrosis transmembrane conductance regulator (CFTR). Recent advances in CF treatment have included use of small-molecule drugs known as modulators, such as Lumacaftor (VX-809), but their detailed mechanism of action and interplay with the surrounding lipid membranes, including cholesterol, remain largely unknown. To examine these phenomena and guide future modulator development, we prepared a set of wild type (WT) and mutant helical hairpin constructs consisting of CFTR transmembrane (TM) segments 3 and 4 and the intervening extracellular loop (termed TM3/4 hairpins) that represent minimal membrane protein tertiary folding units. These hairpin variants, including CF-phenotypic loop mutants E217G and Q220R, and membrane-buried mutant V232D, were reconstituted into large unilamellar phosphatidylcholine (POPC) vesicles, and into corresponding vesicles containing 70 mol% POPC +30 mol% cholesterol, and studied by single-molecule FRET and circular dichroism experiments. We found that the presence of 30 mol% cholesterol induced an increase in helicity of all TM3/4 hairpins, suggesting an increase in bilayer cross-section and hence an increase in the depth of membrane insertion compared to pure POPC vesicles. Importantly, when we added the corrector VX-809, regardless of the presence or absence of cholesterol, all mutants displayed folding and helicity largely indistinguishable from the WT hairpin. Fluorescence spectroscopy measurements suggest that the corrector alters lipid packing and water accessibility. We propose a model whereby VX-809 shields the protein from the lipid environment in a mutant-independent manner such that the WT scaffold prevails. Such ‘normalization’ to WT conformation is consistent with the action of VX-809 as a protein-folding chaperone.
Mathias Schenkel is atCUBE – Center for Molecular Bioengineering, TU Dresden, Tatzberg 41, 01307 Dresden, Germany
Kevin W Southern, Carlo Castellani, Elise Lammertyn, Alan Smyth, Donald VanDevanter, Silke van Koningsbruggen-Rietschel and 36 authors. Standards of care for CFTR variant-specific therapy (including modulators) for people with cystic fibrosis. J Cyst Fibros. 2023 Jan;22(1):17-30. doi: 10.1016/j.jcf.2022.10.002. Epub 2022 Oct 28. Free article pubmed.ncbi.nlm.nih.gov/36916675/

Kevin Southern
Cystic fibrosis (CF) has entered the era of variant-specific therapy, tailored to the genetic variants in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. CFTR modulators, the first variant-specific therapy available, have transformed the management of CF. The latest standards of care from the European CF Society (2018) did not include guidance on variant-specific therapy, as CFTR modulators were becoming established as a novel therapy.
We have produced interim standards to guide healthcare professionals in the provision of variant-specific therapy for people with CF. Here we provide evidence-based guidance covering the spectrum of care, established using evidence from systematic reviews and expert opinion. Statements were reviewed by key stakeholders using Delphi methodology, with agreement (≥80%) achieved for all statements after one round of consultation. Issues around accessibility are discussed and there is clear consensus that all eligible people with CF should have access to variant-specific therapy.
| 1 | For individuals with clinical features consistent with CF, disease-causing variants of the CFTRgene are those characterized as “CF-causing” or “varying clinical consequences” by an established and validated program (for example, CFTR2 or CFTR-France). |
| 2 | Individuals under consideration for CFTR modulator use should have molecular diagnostic testing of the CFTR gene that includes, at minimum, the most frequent variants known to be CF-causing in their population of origin. Further analysis may include exonic regions, intron-exon junctions, and presence of copy number variants, in the case of incomplete genotype after initial molecular testing. |
| 3 | CFTR gene variants should be considered of uncertain clinical significance in the absence of epidemiological or laboratory evidence. These variants should undergo further evaluation to determine their pathogenic or benign status and potential responsiveness to VST. |
| 4 | PwCF aged six years and older, with one or two F508del variants, should have daily treatment with triple modulator therapy (elexacaftor-tezacaftor-ivacaftor). |
| 5 | PwCF and at least one responsive non-F508del variant should be considered for mono (ivacaftor), dual (tezacaftor-ivacaftor) or triple CFTR modulator therapy (elexacaftor-tezacaftor-ivacaftor). |
| 6 | Children with CF with eligible CFTR gene variants should be offered treatment with ivacaftor from 4 months of age. |
| 7 | Children with CF who are homozygous for the F508del variant, aged 2–5 years, should be offered treatment with dual modulator therapy (Lumacaftor-ivacaftor). |
| 8 | Parent/carers of pre-school children with CF should be aware of the efficacy data and safety profile of VST before treatment start. |
| 9 | Before initiating treatment, pwCF and their families should have a detailed discussion with the CF team, outlining the impact of taking CFTR modulator therapy, backed up with written information. |
| 10 | Before starting CFTR modulator therapy, a detailed drug history should be obtained and cross checked with prescribing information about potential drug interactions. |
| 11 | Patients should be followed up at least every 3 months after initiating CFTR modulator therapy to monitor progress and screen for side effects. |
| 12 | CF teams should monitor adherence to CFTR modulator therapy, for example, by using pharmacy dispensing data. |
| 13 | Before commencing and once established on a VST, pwCF, in partnership with the physiotherapy team, may need to adapt and optimise their airway clearance technique and sinus treatments. |
| 14 | For pwCF starting a VST, the management of CFRD should be reviewed and adapted on an individual basis, considering clinical and nutritional status. |
| 15 | PwCF on VST should continue to receive regular monitoring of nutritional status and dietary intake, according to changing energy requirements. |
| 16 | Frequency of support of nutritional assessment should be individualized, depending on age, clinical status and CFTR modulator therapy. |
| 17 | CF teams should be familiar with the wide-ranging psychological impact of VST and prepare, advise and support pwCF and their caregivers as required, involving the CF psychologist when indicated. |
| 18 | Symptoms of depression and anxiety should be assessed pre-VST and no later than 3 months after starting. |
| 19 | Prior to initiating VST in women with CF, contraception and fertility should be reassessed, and appropriate counselling provided. |
| 20 | The decision to use VST during pregnancy should weigh the risk to maternal health in the event of withholding therapy and the lack of data regarding safety to the foetus. |
| 21 | Women treated with VST planning to breastfeed should be informed regarding lack of data on safety during breastfeeding. |
| 22 | PwCF and CFTR gene variant(s) with unclear response to modulator therapy should be offered referral to a centre with capacity for ex vivo testing of CFTR response, to potentially establish an individualized treatment plan, including with modulator therapies. |
| 23 | All pwCF with non-responsive CFTR gene variants should continue to receive high-quality CF-specialist multi-disciplinary care at a specialist or accredited CF centre. |
| 24 | It is important that pwCF and non-responsive CFTR gene variants are informed of clinical trials and supported to participate in trials. |
| 25 | For pwCF after solid organ transplant, VST should be considered for eligible patients after discussion of the potential risks and benefits between the patient, the CF team, and the transplant team. |
| 26 | For patients with a diagnosis of CFTR-related disorder, there is no evidence to support the use of VST outside of clinical trials. |
| 27 | For infants with an unclear diagnosis following newborn screening for CF (CRMS/CFSPID), the use of VST is not indicated. |
| 28 | For new high-cost CF therapies, a robust health technology assessment should evaluate the impact on pwCF and society. |
| 29 | Evidence used to inform reimbursement decisions using public money should be transparent and available to the public. |
| 30 | The CF community should advocate globally for equitable access to new therapies with proven efficacy for all pwCF. |
– In this rapidly changing area of CF treatment this is a clear article with agreed consensus guidance on the use of the new modulators. The full article is available via the above PubMed link; it is excellent and throughly recommended.
Kevin W Southern , Jürg Barben, Stephen Goldring, Rachel Kneen, Suzanne Southward, Yashasvi Rajeev, Jane C Davies, Andrew Bush. Raised Intracranial Pressure in Three Children with Cystic Fibrosis Receiving Elexacaftor-Tezacaftor-Ivacaftor Modulator Therapy. Am J Respir Crit Care Med
. 2023 May 9. doi: 10.1164/rccm.202303-0380LE. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37159937/
Prof. Kevin Southern is at the University of Liverpool, Women’s and Children’s Health, Liverpool, Merseyside, United Kingdom of Great Britain and Northern Ireland; kwsouth@liv.ac.uk.
Mirjam Stahl , Jobst Roehmel, Monika Eichinger , Felix Doellinger , Lutz Naehrlich , Matthias V Kopp , Anna-Maria Dittrich , Christopher Lee, Olaf Sommerburg , Simon Tian , Tu Xu , Pan Wu, Aniket Joshi, Partha Ray , Margaret E Duncan , Mark O Wielpütz, Marcus A Mall Effects of Lumacaftor/Ivacaftor on Cystic Fibrosis Disease Progression in Children 2 through 5 Years of Age Homozygous for F508del-CFTR: A Phase 2 Placebo-controlled Clinical Trial. Ann Am Thorac Soc
. 2023 Mar 21. doi: 10.1513/AnnalsATS.202208-684OC. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36943405/

Mirjam Stahl
Rationale: Lumacaftor/ivacaftor (LUM/IVA) was shown to be safe and well tolerated in children 2 through 5 years of age with cystic fibrosis (CF) homozygous for F508del-CFTR in a phase 3 open-label study. Improvements in sweat chloride concentration, markers of pancreatic function, and lung clearance index2.5 (LCI2.5), along with increases in growth parameters, suggested the potential for early disease modification with LUM/IVA treatment.
Objective: To further assess the effects of LUM/IVA on CF disease progression in children 2 through 5 years of age using chest magnetic resonance imaging (MRI).
Methods: This phase 2 study had two parts: a 48-week, randomized, double-blind, placebo-controlled treatment period in which children 2 through 5 years of age with CF homozygous for F508del-CFTR received either LUM/IVA or placebo (Part 1) followed by an open-label period in which all children received LUM/IVA for an additional 48 weeks (Part 2). We report results from Part 1. The primary endpoint was absolute change from baseline in chest MRI global score at Week 48. Secondary endpoints included absolute change in LCI2.5 through Week 48 and absolute changes in weight-for-age, stature-for-age, and body mass index-for-age z-scores at Week 48. Additional endpoints included absolute changes in sweat chloride concentration, fecal elastase-1 levels, serum immunoreactive trypsinogen, and fecal calprotectin through Week 48. The primary endpoint was analyzed using Bayesian methods, where the actual Bayesian posterior probability of LUM/IVA being superior to placebo in the MRI global chest score at Week 48 was calculated using a vague normal prior distribution; secondary and additional endpoints were analyzed using descriptive summary statistics.
Results: Fifty-one children were enrolled and received LUM/IVA (n=35) or placebo (n=16). For the change in MRI global chest score at Week 48, the Bayesian posterior probability of LUM/IVA being better than placebo (treatment difference <0; higher score indicating greater abnormality) was 76%; the mean treatment difference was -1.5 (95% credible interval, -5.5 to 2.6). Treatment with LUM/IVA also led to within-group numerical improvements in LCI2.5, growth parameters, and biomarkers of pancreatic function as well as greater decreases in sweat chloride concentration compared with placebo from baseline through Week 48. Safety data were consistent with the established safety profile of LUM/IVA.
Conclusions: This placebo-controlled study suggests the potential for early disease modification with LUM/IVA treatment, including that assessed by chest MRI, in children as young as 2 years. Clinical trial registered with ClinicalTrials.gov (NCT03625466).
Mirjam Stahl is at the Charité Universitätsmedizin Berlin – Campus Virchow-Klinikum, 72217, Department of Pediatric Pulmonology, Immunology and Critical Care Medicine, Berlin, Germany; German Center for Lung Research (DZL), Berlin, Germany; Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Berlin, Germany.
Carmen Streibel, Corin C Willers , Orso Pusterla , Grzegorz Bauman, Enno Stranzinger, Ben Brabandt , Oliver Bieri, Marion Curdy, Marina Bullo, Bettina Sarah Frauchiger , Insa Korten, Linn Krüger, Carmen Casaulta, Felix Ratjen, Philipp Latzin, Elisabeth Kieninger. Effects of elexacaftor/tezacaftor/ivacaftor therapy in children with cystic fibrosis – a comprehensive assessment using lung clearance index, spirometry, and functional and structural lung MRI . J Cyst Fibros. 2023 Jan 10;S1569-1993(22)01432-1. doi: 10.1016/j.jcf.2022.12.012. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36635199/

Carmen Streibel
Background: With improvement in supportive therapies and the introduction of cystic fibrosis transmembrane conductance regulator (CFTR)-modulator treatment in patients with cystic fibrosis (CF), milder disease courses are expected. Therefore, sensitive parameters are needed to monitor disease course and effects of CFTR-modulators. Functional lung MRI using matrix-pencil decomposition (MP-MRI) is a promising tool for assessing ventilation and perfusion quantitatively. This study aimed to assess the treatment effect of elexacaftor/tezacaftor/ivacaftor combination regimen (ELX/TEZ/IVA) on measures of structural and functional lung abnormalities.
Methods: 24 children with CF underwent lung function tests (multiple breath washout, spirometry), functional and structural MRI twice (one year apart) before and once after at least two weeks (mean 4.7 ± 2.6 months) on ELX/TEZ/IVA. Main outcomes were changes (Δ) upon ELX/TEZ/IVA in lung function, defect percentage of ventilation (VDP) and perfusion (QDP), defect distribution index of ventilation and perfusion (DDIV, DDIQ), and Eichinger score. Statistical analyses were performed using paired t-tests and multilevel regression models with bootstrapping.
Results: We observed a significant improvement in lung function, structural and functional MRI parameters upon ELX/TEZ/IVA treatment (mean; 95%-CI): ΔLCI2.5 (TO) -0.84 (-1.62 to -0.06); ΔFEV1 (z-score) 1.05 (0.56 to 1.55); ΔVDP (% of impairment) -6.00 (-8.44 to -3.55); ΔQDP (% of impairment) -3.90 (-5.90 to -1.90); ΔDDIV -1.38 (-2.22 to -0.53); ΔDDIQ -0.31 (-0.73 to 0.12); ΔEichinger score -3.89 (-5.05 to -2.72).
Conclusions: Besides lung function tests, functional and structural MRI is a suitable tool to monitor treatment response of ELX/TEZ/IVA therapy, and seems promising as outcome marker in the future.
Carmen Streibel is in the Dept of Paediatric Respiratory Medicine and Allergology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Switzerland.Division of Paediatric Respiratory Medicine and Allergology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Switzerland.
P Tachtatzis, G Spoletini, I Clifton, C Etherington, D Peckham. Changes in liver biochemistry and tacrolimus levels following the introduction of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and liver transplant.J Cyst Fibros. 2023 May 8;S1569-1993(23)00129-7. doi: 10.1016/j.jcf.2023.04.023. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37164896/
Introduction: Elevated liver function tests (LFTs) are reported in individuals with cystic fibrosis (CF) starting elexacaftor/tezacaftor/ivacaftor (ETI). We report our experience with ETI in CF liver transplant patients.
Method: All CF liver transplant patients under the care of the Leeds CF team were commenced on ETI. Liver biopsies were performed when ALT >3 times upper limit of normal with or without bilirubin elevation. Treatment was guided by transplant hepatology and CF teams. Clinical data including lung function, LFTs and tacrolimus levels were collected.
Results: Four patients (3 male, 1 female) on tacrolimus were commenced on ETI. Median time post liver transplantation was 6.5 years. Three patients underwent liver biopsy. One biopsy was abnormal with immune-mediated liver injury, which responded to increased immunosuppression. Management of tacrolimus levels proved straightforward.
Conclusion: ETI therapy in CF post liver transplant recipients was encouraging. Normal liver biopsy provides re-assurance to continue treatment despite elevated LFTs.
Dr Phaedra Tachtatzis is consultant hepatologist at Liver Transplantation & Hepatology, Leeds Teaching Hospitals NHS Trust, Leeds, UK; Regional Adult Cystic Fibrosis Unit, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Daniel H Tewkesbury, Varinder Athwal, Rowland J Bright-Thomas, Andrew M Jones, Peter J Barry Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on liver tests at a large single adult cystic fibrosis centre. J Cyst Fibros. 2023 Jan 18;S1569-1993(23)00008-5. doi: 10.1016/j.jcf.2023.01.007. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36669962/
Background: Elexacaftor/tezacaftor/ivacaftor (E/T/I) therapy has resulted in substantial improvements in health status for many with cystic fibrosis. Monitoring of liver tests is recommended due to observed rises in transaminases in trials and cases of hepatotoxicity. Comprehensive data in large populations of unselected individuals and those with established CF related liver disease (CFLD) is lacking.
Methods: Patients prescribed E/T/I at a large, adult centre had liver tests monitored at least 3 monthly for 12 months. Changes in individual liver tests were analysed and abnormalities were compared in those with and without CFLD.
Results: 255 of 267 eligible patients were included. Mild rises in median ALT, AST and bilirubin from baseline to 3 months (all p < 0.001) within normal limits were noted which were sustained. There were no differences in changes in liver tests between those with or without CFLD. There was a significant difference in alkaline phosphatase for those with raised levels at baseline versus those with normal baseline level (-18.5 vs +2.0 IU/L, p = 0.002). Clinically significant rises in ALT and AST occurred in 8 (3.1%) and 6 (2.4%) cases respectively, with derangements in 2 individuals attributed to therapy.
Conclusions: E/T/I leads to a mild, likely clinically insignificant increase in ALT, AST and bilirubin after 3 months which is sustained but does not appear to increase further in the vast majority. Underlying CFLD should not be a barrier to treatment. Although there was a reduction in ALP when elevated at baseline, this was not unique to those with pre-existing CFLD.
Daniel H Tewkesbury is at the Manchester University NHS Foundation Trust, Manchester, UK; Division of Immunology, Immunity to Infection & Respiratory Medicine, University of Manchester, Manchester, UK.
Burhard Tummler.Post-approval studies with the CFTR modulators Elexacaftor-Tezacaftor-Ivercaftor. Front Pharmacol. 2023 Mar 21;14:1158207. doi: 10.3389/fphar.2023.1158207. eCollection 2023. pubmed.ncbi.nlm.nih.gov/37025483/ Free PMC article

Burkhard Tümmler
Medizinische Hochschule
Hannove
Triple combination therapy with the CFTR modulators elexacaftor (ELX), tezacaftor (TEZ) and ivacaftor (IVA) has been qualified as a game changer in cystic fibrosis (CF). We provide an overview of the body of literature on ELX/TEZ/IVA published between November 2019 and February 2023 after approval by the regulators. Recombinant ELX/TEZ/IVA-bound Phe508del CFTR exhibits a wild type conformation in vitro, but in patient’s tissue a CFTR glyoisoform is synthesized that is distinct from the wild type and Phe508del isoforms. ELX/TEZ/IVA therapy improved the quality of life of people with CF in the real-life setting irrespective of their anthropometry and lung function at baseline. ELX/TEZ/IVA improved sinonasal and abdominal disease, lung function and morphology, airway microbiology and the basic defect of impaired epithelial chloride and bicarbonate transport. Pregnancy rates were increasing in women with CF. Side effects of mental status changes deserve particular attention in the future.. Burkhard Tümmler is at the Dept. of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany and the Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research, Hannover Medical School, Hannover, Germany.
— This is a comprehensive review of the post approved studies on published work up to the present. The free article is recommended.
Uluer AZ; MacGregor G; Azevedo P; Indihar V; Keating C; Mall MA; McKoneEF; Ramsey BW; Rowe SM; Rubenstein RC; Taylor-Cousar JL; Tullis E; Yonker LM; Chu C; Lam AP; Nair N; Sosnay PR; Tian S; Van Goor F; Viswanathan L; Waltz D; Wang LT; Xi Y; Billings J; Horsley A. Safety and efficacy of vanzacaftor-tezacaftor-deutivacaftor in adults with cystic fibrosis: randomised, double-blind, controlled, phase 2 trials. The Lancet Respiratory Medicine. 11(6):550-562, 2023 06. Free article pubmed.ncbi.nlm.nih.gov/36842446/

Ahmet Uluer
BACKGROUND: Elexacaftor-tezacaftor-ivacaftor has been shown to be safe and efficacious in people with cystic fibrosis and at least one F508del allele. Our aim was to identify a novel cystic fibrosis transmembrane conductance regulator (CFTR) modulator combination capable of further increasing CFTR-mediated chloride transport, with the potential for
once-daily dosing.
METHODS and FINDINGS are included in the PubMed link
INTERPRETATION: Once-daily dosing with vanzacaftor-tezacaftor-deutivacaftor was safe and well tolerated and improved lung function, respiratory symptoms, and CFTR function. These
results support the continued investigation of vanzacaftor-tezacaftor-deutivacaftor in phase 3 clinical trials compared with elexacaftor-tezacaftor-ivacaftor.
Dr Ahmet Z.Ukuer is at the Boston Children’s Hospital, Harvard Medical School,
Boston, MA, USA; Brigham & Women’s Hospital CF Center, Boston, MA, USA.
Claire Wainwright, Susanna A McColley, Paul McNally , Michael Powers, Felix Ratjen , Jonathan H Rayment , George Retsch-Bogart , Erica Roesch , Neil Ahluwalia , Anna Chin , Chenghao Chu, Mengdi Lu, Prema Menon, David Waltz 11, Tanya Weinstock, Laura Zelazoski, Jane C Davies; VX19-445-107 Study Group. Long-Term Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged ≥6 Years with Cystic Fibrosis and At Least One F508del Allele: A Phase 3, Open-Label Clinical Trial. Am J Respir Crit Care Med
. 2023 May 8. doi: 10.1164/rccm.202301-0021OC. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37154609/

Claire Wainwright
Rationale: A 24-week, phase 3, open-label study showed elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was safe and efficacious in children aged 6 through 11 years with cystic fibrosis and ≥1 F508del-CFTR allele.
Objectives: To assess long-term safety and efficacy of ELX/TEZ/IVA in children who completed the pivotal 24-week phase 3 trial.
Methods: In this phase 3, two-part (Part A and Part B), open-label extension study, children aged ≥6 years with cystic fibrosis heterozygous for F508del and a minimal function CFTR mutation (F/MF genotypes) or homozygous for F508del (F/F genotype) who completed the 24-week parent study received ELX/TEZ/IVA based on weight. Children weighing <30 kg received ELX 100 mg once daily/TEZ 50 mg once daily/IVA 75 mg every 12 hours while children weighing ≥30 kg received ELX 200 mg once daily/TEZ 100 mg once daily/IVA 150 mg every 12 hours (adult dose). The 96-week analysis of Part A of this extension study is reported here.
Measurements and main results: Sixty-four children (F/MF genotypes, n=36; F/F genotype, n=28) were enrolled and received ≥1 dose of ELX/TEZ/IVA. Mean (SD) period of exposure to ELX/TEZ/IVA was 93.9 (11.1) weeks. The primary endpoint was safety and tolerability. Adverse events and serious adverse events were consistent with common manifestations of cystic fibrosis disease. Overall, exposure-adjusted rates of adverse events and serious adverse events (407.74 and 4.72 events per 100 patient-years) were lower than in the parent study (987.04 and 8.68 events per 100 patient-years). One child (1.6%) had an adverse event of aggression that was moderate in severity and resolved after study drug discontinuation. From parent study baseline at Week 96 of this extension study, mean ppFEV1 increased (11.2 [95% CI 8.3, 14.2] percentage points), sweat chloride concentration decreased (-62.3 [95% CI 65.9, -58.8] mmol/L), Cystic Fibrosis Questionnaire-Revised respiratory domain score increased (13.3 [95% CI 11.4, 15.1] points), and LCI2.5 decreased (-2.00 [95% CI -2.45, -1.55] units). Increases in growth parameters were also observed. The estimated pulmonary exacerbation rate per 48 weeks was 0.04. The annualized rate of change in ppFEV1 was 0.51 (95% CI -0.73, 1.75) percentage points per year.
Conclusions: ELX/TEZ/IVA continued to be generally safe and well tolerated in children aged ≥6 years through an additional 96 weeks of treatment. Improvements in lung function, respiratory symptoms, and CFTR function observed in the parent study were maintained. These results demonstrate the favorable long-term safety profile and durable clinical benefits of ELX/TEZ/IVA in this pediatric population. Clinical trial registration available at www. Clinicaltrials: gov, ID: NCT04183790.
Prof. Claire Wainwright is at the Royal Children’s Hospital, Respiratory Medicine, Brisbane, Queensland, Australia
Angela Wang, MinJae Lee, Ashley Keller, Sarah Jian, Karen Lowe, James D Finkle , Raksha Jain Sex differences in outcomes of people with cystic fibrosis treated with elexacaftor/tezacaftor/ivacaftor. J Cyst Fibros. 2023 May 25;S1569-1993(23)00139-X. doi: 10.1016/j.jcf.2023.05.009. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37244841/
Background: There is a well described sex-disparity in outcomes of individuals with cystic fibrosis (CF), with females faring worse than males. Given the dramatic improvement in overall health of people with CF using CF transmembrane conductance regulator (CFTR) modulator therapy, elexacaftor/tezacaftor/ivacaftor (ETI), the sex-disparity in CF warrants re-examination.
Methods: We evaluated the effects of ETI use by sex prior to versus after initiation of ETI by pulmonary exacerbations (PEx), percent predicted forced expiratory volume in one second (ppFEV1), presence of Pseudomonas aeruginosa in sputum cultures, and body mass index (BMI). We used univariate and multivariable longitudinal regression adjusting for key confounders, such as age, race, CFTR modulator taken prior to ETI and baseline ppFEV1.
Results: We included 251 individuals started on ETI between January 2014 to September 2022. We collected data for a mean of 5.45 years pre-ETI and 2.38 years post-ETI. We found the adjusted presence of PEx decreased more in males than females pre- to post-ETI with the odds of having a PEx in males being 0.57 (43% reduction) versus females 0.75 (25% reduction) (p = 0.049). We found no statistical difference by sex for ppFEV1, presence of Pseudomonas aeruginosa or BMI pre- to post-ETI by sex.
Conclusion: After treatment with ETI, there was a greater decline in PEx in males versus females. Long-term impact of ETI by sex is still unknown, but we will need to seek ways to effectively tailor care for individuals with CF and consider pharmacokinetic studies of ETI comparing males to females.
Angela Wang is at the School of Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA.
J Westhoff, S Barth, L Naehrlich. Drug desensitization to lumacaftor/ivacaftor: A fast lane to drug tolerance. Case Reports J Cyst Fibros
. 2023 Apr 10;S1569-1993(23)00079-6. doi: 10.1016/j.jcf.2023.03.015. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37045685/
We present the case of a girl (now 11 years and 9 months old) with cystic fibrosis (F508del homozygote), who developed pruritic rash and urticaria six days after the first dose of the CFTR modulators lumacaftor/ivacaftor. The treatment was paused and had to be interrupted due to an immediate recurrence of the urticarial rash after rechallenge. We developed a drug desensitization protocol, aligned to protocols used for desensitization against oral antibiotics. In contrast to other published protocols, it was performed by rapidly increasing the dose of lumacaftor/ivacaftor granulate at 15 min intervals. The medication was continued without interruption, the rash did not reappear during follow-up of two years. This drug desensitization protocol provides a potential new therapeutic option for patients with drug hypersensitivity reactions to CFTR modulators, especially when there are no alternative treatments. Lumacaftor/ivacaftor is available as granulate, doses can be titrated during desensitization and used for long-term treatment.
Julia Westoff is in the Department of Pediatrics, Pediatric Pulmonology, Justus-Liebig-University Giessen, Germany.
Vered Wiesel , Micha Aviram , Meir Mei-Zahav, Miri Dotan, Dario Prais , Malena Cohen-Cymberknoh, Michal Gur, Ronen Bar-Yoseph , Galit Livnat , Aviv Goldbart , Guy Hazan , Itai Hazan , Inbal Golan-Tripto. Eradication of Nontuberculous Mycobacteria in People with Cystic Fibrosis Treated with Elexacaftor/Tezacaftor/Ivacaftor: A Multicenter Cohort Study. J Cyst Fibros. 2023 May 10;S1569-1993(23)00133-9. doi: 10.1016/j.jcf.2023.05.003. Online ahead of print. pubmed.ncbi.nlm.nih.gov/37173154/
Background: The prevalence of nontuberculous mycobacteria (NTM) infections is rising in people with cystic fibrosis (pwCF). NTM infection, especially infection with Mycobacterium abscessus complex (MABC), is commonly associated with severe lung deterioration. The current treatment modalities, including multiple intravenous antibiotics, frequently fail to achieve airway eradication. Although treatment with elexacaftor/tezacaftor/ivacaftor (ETI) has been shown to modulate the lung microbiome, data regarding its role in eradicating NTM in pwCF is lacking. Our aim was to evaluate the impact of ETI on the rate of NTM eradication in pwCF.
Methods: This retrospective multicenter cohort study included pwCF from five CF centers in Israel. PwCF aged older than 6 who had at least one positive NTM airway culture in the past two years and were treated with ETI for at least one year were included. The annual NTM and bacterial isolations, pulmonary function tests, and body mass index were analyzed before and after ETI treatment.
Results: Fifteen pwCF were included (median age 20.9 years, 73.3% females, 80% pancreatic insufficient). In nine patients (66%) NTM isolations were eradicated following treatment with ETI. Seven of them had MABC. The median time between the first NTM isolation and treatment with ETI was 2.71 years (0.27-10.35 years). Eradication of NTM was associated with improved pulmonary function tests (p<0.05).
Conclusions: For the first time, we report successful eradication of NTM, including MABC, following treatment with ETI in pwCF. Additional studies are needed to assess whether treatment with ETI can result in the long-term eradication of NTM.
Vered Wiesel is at the Medical School for International Health, Ben Gurion University, Beer Sheva, Israel; Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel.
