2006

Aird FK, Greene SA, Ogston SA, Macdonald TM, Mukhopadhyay S. Vitamin A and lung function in CF. J Cyst Fibros 2006; 5:129-131. pubmed.ncbi.nlm.nih.gov/16650745/

Fig. 1 Somnath-Mukhopadhyay
bsms.ac.uk
Laboratory evidence suggests that vitamin A could have a protective effect on respiratory status in patients with cystic fibrosis (CF). This study shows a significant correlation between serum vitamin A concentrations and every aspect of lung function tested in 38 patients with stable CF. Serum vitamin D and vitamin E concentrations were also measured but did not show any significant correlations with lung function.
– This is interesting as Dorothy Andersen initially likened the changes in the bronchial epithelium to the epithelial metaplasia found in vitamin A deficiency and for some years considered that the pulmonary problems were related to these changes in the bronchial epithelium rendering them susceptible to infection – i.e. she believed the condition was primarily the result of primary intestinal malabsorption resulting in vitamin deficiency. The authors of the present paper note this is the first study showing a definite relationship between vitamin A levels and respiratory function tests in CF.
Dr Fiona K Aird is in the Division of Maternal and Child Health Sciences, University of Dundee, Ninewells Hospital and Medical School, Scotland,
Dr Samnath Mukhopadhyay (fig.1) at the time of this report was Senior Clinical Lecturer and Hon. Consultant in Dundee Medical School; from 2007 he was Professor of Paediatrics at Brighton and Sussex Medical School.
Andersen HU, Lanng S, Pressler T, Laugesen CS, Mathiesen ER. Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006; 29:2660-3. [PubMed]

Fig. 2 Henrik Ullits Andersen Copenhagen University Hospitals
Thirty-eight patients with an average age of 30 years (range 18-55yrs) with CF-related diabetes for 20 years (0-31yrs) were screened for diabetes complications at the Copenhagen CF Centre. Because of chronic pulmonary infections, the majority of patients were regularly treated with aminoglycoside and cyclosporine was given to those who had lung transplants. Since the pharmacological treatment of lung transplant patients could influence metabolic regulation and renal function, the results were given separately for nontransplanted (n = 29) and transplanted (n = 9) CF patients.
Nine of these diabetic patients (27%) had retinopathy, two of whom had proliferative retinopathy requiring laser treatment. Lung transplantation did not affect the prevalence of retinopathy. In 29 non-transplanted patients, nine had hypertension, three microalbuminuria, and one elevated creatinine; none had macroalbuminuria. In transplanted patients (9), eight had hypertension, two had microalbuminuria, and none had macroalbuminuria; seven of the nine had elevated plasma creatinine, and severely reduced glomerular filtration rate was significantly more frequent.
A high frequency of diabetic retinopathy was found in patients with insulin-treated CF-related diabetes, stressing the need for a regular screening program as in type 1 diabetes. Severely impaired kidney function was common in lung transplant patients, probably in part secondary to cyclosporine treatment but many would also have had life long regular courses of intravenous aminoglycosides. As age increases so do complications. Eventually the majority of older people with CF will develop diabetes and it is clear that eventually many will develop diabetic complications particularly retinopathy. The added complications associated with immunosuppressant drugs in those who have had lung transplants and the effects of repeated courses of aminoglycosides contribute to the high frequency of renal complications.
Henrik Ullits Andersen (fig. 2) is Chief Consultant in the Department of Endocrinology, Rigshospitalet, State University Hospital, Blegdamsvej, DK-2100 Købehavn Ø, Denmark.
Asherova IK, Feigelson J, Vasilyeva LA, Gabitov VJ. Cystic fibrosis complicated by multiresistant tuberculosis. Acta Paediatrica 2006; 95:1513-4. [PubMed]

Fig 3. Jean Feigelson. Author’s photo in 2006.
Tuberculosis is a rare occurrence in CF. The second author is Jean Feigelson of Paris (1921-2017) who published his first paper on CF in 1963 ([PubMed]).
Jean Feigelson (1921-2017) (fig. 3) of Paris is one of the pioneers of CF care and still a regular attendee at CF conferences in Europe and North America. He trained in paediatrics at the Sick Children’s Clinic in Oslo in 1952. In a career spanning over 45 years he has treated over 250 people with cystic fibrosis. This is Jean Feigelson’s first recorded paper on cystic fibrosis. His most recent is as a co-author of a paper on partial splenectomy, that was published in 2007 (Louis D et al, Pediatr Pulmonol 2007; 42:1173-1180). He has 48 references noted in Medline produced steadily over 40 years
“Jean Feigelson was a member of the French Resistance, war hero, Knight of the national order of the Legion of Honour, and holder of many other awards; in the times of peace he continued his fight against any injustice. He inspired doctors to fight for people suffering from cystic fibrosis, so that they could have a long and fulfilled life. He filled people with energy, self-confidence, and made them believe in the scientific potential of medicine”.
Balfour-Lynn IM, Lees B, Hall P, Phillips G, Kahn M, Flather M, Elborn JS. On behalf of the WISE investigators. Multicenter randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. Am J Respir Crit Care Med 2006; 173:1356-1362. [PubMed]

Fig. 4 Ian Balfour-Lynn Brompton Hospital
On the grounds that the authors considered that inhaled steroids are widely used despite lack of evidence, this study was to test the safety of withdrawal of inhaled corticosteroids with the hypothesis this would not be associated with an earlier onset of acute chest exacerbations. Patients during the 2-month run-in period, received fluticasone; they then took either fluticasone or placebo for 6 months. There was no difference in time to first exacerbation in the two groups. So in this study population (applicable to a surprising 40% of patients with cystic fibrosis in the UK), it appears safe to consider stopping inhaled corticosteroids.
– However, it would be important to consider the individual patient’s clinical history prior to their starting inhaled steroids before considering withdrawing the treatment. Note that the summary does not mention the 24 patients whose steroids their clinicians were unwilling to stop. They had more asthma, more were atopic and they had more exacerbations and were in a worse condition. In this reviewer’s experience, many of these patients are considerably, even dramatically, improved when they commence inhaled steroids. Trials of N=1 are obviously useful in this clinical situation.
Dr Ian Balfour-Lynn (fig.4) is consultant paediatrician at the Royal Brompton Hospital, London. He is involved in many areas of both patient care and CF research.
Davidson H, McLachlan G, Wilson A, Boyd AC, Doherty A, MacGregor G, Davies L. Painter HA, Coles R, Hyde SC, Gill DR, Amaral MD, Collie DD, Porteous DJ, Penque D. Human-specific cystic fibrosis transmembrane conductance regulator antibodies detect in vivo gene transfer to ovine airways. Am J Resp Cell Mol 2006; 35:72-83. [PubMed]
A study from the UK Gene Therapy Consortium using sheep in their gene therapy research confirming the sheep as being a suitable model in terms of size for studies on delivery of nebulised material to the airways and transfection.This is possible in sheep, as although they do not have CF, the human and ovine CFTR can be identified in the airway cells and differentiated.
Heather Davidson is at Medical Sciences (Medical Genetics), University of Edinburgh, Molecular Medicine Centre, Western General Hospital, Edinburgh EH4 2XU, United Kingdom.
Dellon ES, Morgan DR, Mohanty SP, Davis K, Aris RM. High incidence of gastric bezoars in cystic fibrosis patients after lung transplantation. Transplantation 2006; 81:1141-6. pubmed.ncbi.nlm.nih.gov/16641599/
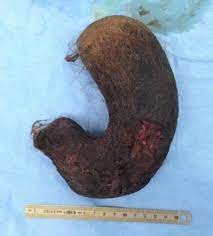
Fig. 3 Gastric Bezoar
Radiopaedia

Fig. 5 Evan S Dellon
med.unc.edu
A retrospective analysis of patients who underwent lung transplantation from December, 1992 through July, 2005 at a tertiary care medical center. Of the 215 patients who received lung transplantation, 17 (7.9%) developed gastric bezoars confirmed by upper endoscopy. (A bezoar results from the accumulation of ingested material in the form of masses or concretions). Cystic fibrosis was the leading indication for lung transplantation (n=145), and 11% of cystic fibrosis patients (16 of 145) formed gastric bezoars after transplant. Additionally, 94% of patients with bezoars (16 of 17) had cystic fibrosis (P=0.02), with the exception being a subject with primary ciliary dyskinesia. No patient who underwent lung transplant for another indication was found to have a bezoar. The mean time to diagnosis was 34 days, with two-thirds of bezoars diagnosed within one month after transplant. The annual incidence was unchanged during the study period.
Gastric bezoars are surprisingly common in cystic fibrosis patients after lung transplantation. The etiology is likely multifactorial, related to gastric motility, respiratory secretions, and medications. Further investigation is needed to understand the pathogenesis of bezoar formation in this selected population, and strategies for primary prevention may be beneficial. This complication seems to be relatively common after transplants in people with CF who are known to have abnormal gastric motility.
Dr Evan S Dellon (Fig.5) is a Professor in the Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC
Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. National Hypertonic Saline in Cystic Fibrosis Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Eng J Med 2006; 354:229-240. [PubMed]

Fig.6 Peter Bye and Kingsley Coultard
Inhaled hypertonic saline (7%) acutely increases mucociliary clearance and, in short-term trials, improves lung function in people with cystic fibrosis. Peter Bye (fig. 6) of St Vincent’s Hospital Sydney, and Mark Elkins (fig. 7) and their colleagues tested the safety and efficacy of inhaled hypertonic saline after a bronchodilator in a long-term double-blind, parallel-group trial over 48 weeks. The rate of change (slope) in lung function did not differ significantly between the treated and control groups but the absolute difference in lung function between groups was significant (P=0.03) when averaged across all post-randomization visits. The hypertonic-saline group also had a significant 56% reduction in pulmonary exacerbations. This Australian study was funded by the US CF Foundation and received considerable attention.
A similar short term study from Chapel Hill, USA showed similar benefit in mucus clearance and FEV1 (6.62% increase) but not when used with amiloride which was considered to inhibit osmotically driven water transport (Donaldson SH et al. NEJM 2006; 354:241-250. [PubMed]).

Fig. 7 Mike Elkins
Mark Elkins (fig. 7) is Clinical Associate Professor at the Royal Prince Albert Hospital, The University of Sydney.
Subsequently 7% saline was shown to be tolerated by infants with CF when assessed by infant respiratory function tests and clinically (Subbarao P et al, Pediatr Pulmonol 2007; 42:471-476. [PubMed]) although in children less than 6 years 48 weeks of hypertonic saline compared with isotonic saline did not reduce the rate of pulmonary exacerbations (Rosenfield M et al. JAMA 2012; 307:2269-2277. [PubMed]).
– The great interest in these findings stemmed partially from the fact that here was a way of correcting the low salt situation in the airways, postulated by Boucher to be a major cause of the fluid deficit and airway mucociliary clearance problems in CF. Also the treatment is inexpensive compared with other very expensive mucolytics such as rhDNase!!
Festini F, Buzzetti R, Bassi C, Braggion C, Salvatore D, Taccetti G Mastella G. Isolation measures for prevention of infection with respiratory pathogens in cystic fibrosis: a systematic review. J Hosp Infect 2006; 64:1-6. [PubMed]

Fig. 8 Gianni Mastella
The aim of this review was to determine the evidence available to support the efficacy of isolation (or segregation) practices in preventing, delaying or reducing the risk for CF patients of acquiring P. aeruginosa and B. cepacia. In the absence of studies with an experimental, controlled design, the efficacy of isolation practices in preventing the transmission of respiratory pathogens in CF remains unproven. However, it is no surprise that the observational studies reviewed seem to support the implementation of isolation (or segregation) measures to reduce the risk of transmission of B. cepacia and P. aeruginosa in CF patients
– There is now certainly enough published evidence to support patient segregation according to microbiological status and a controlled trial would be unethical. The delay in introducing patient segregation and the occurrence of cross infection in some CF centres has provided adequate information that the practice is fully justified!!!
Festini F, Ciuti R, Taccetti G, Repetto T, Campana S, De Martino M. Breast-feeding in a woman with cystic fibrosis undergoing antibiotic intravenous treatment. J Mater-Fetal Neo M 2006; 19:375-376.[PubMed]

Fig. 9 Filippo Festini
A report of a 30-year-old woman with cystic fibrosis (CF) chronically infected with Pseudomonas aeruginosa who delivered and breast-fed a healthy boy. While breast-feeding the woman had to undergo an intravenous antibiotic course with tobramycin, due to pulmonary exacerbation. Tobramycin was not detected in her milk and lactation could be continued.This is the first time that the presence of tobramycin in the milk of a CF woman during i.v. administration has been investigated.
– This is useful information even though only an isolated case report – particularly as pregnancy becomes more common in women with CF and treatment with IV tobramycin may be required in the postnatal period.
Filippo Festini (Fig. 9) is Professor of Nursing Science, University of Florence, Italy.
Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatrica 2006; 95:1070-1074. [PubMed]

Fig. 10 Birgitte Frederiksen & Niels Hoiby. Author’s photo
During one year patients with CF were randomized either to aerosolized Pulmozyme daily or to no Pulmozyme. The number of positive respiratory cultures was higher in the untreated group (82%) compared with the treated group (72%) (p<0.05). The most striking difference was found for Staphylococcus aureus, with a prevalence of 30% in the untreated group compared with 16% in the treated group (p<0.0001). Pulmonary function (FEV1) in the treated group showed a significant increase of 7.3% compared to 0.9% in the untreated group (p<0.05). Long term DNase treatment was beneficial to CF patients without chronic lower respiratory tract infection, with fewer positive respiratory cultures, leading to reduced demand for antibiotics and to improved lung function.Experience from Copenhagen showing inhaled rhDNase reduced the likelihood of a positive respiratory culture. This finding provides additional support for starting all patients on the treatment at an early age, where inhaled treatment is practical, as occurs now in some centres.
Dr Birgitte Frederiksen and Prof. Niels Hoiby (Fig.10) are at the Department of Paediatrics, Copenhagen Cystic Fibrosis Centre, Copenhagen University Hospital (Rigshospitalet), Denmark.
Garwood S, Flume PA, Ravenel J. Superior vena cava syndrome related to indwelling intravenous catheters in patients with cystic fibrosis. Pediatr Pulmonol 2006; 41:683-687. [PubMed]

Fig.11 Susan Garwood
LinkedIn
Three patients with CF had superior vena cava syndrome (thrombosis in the large vein entering the heart) due to the presence in the vessel of a foreign body i.e. the implantable venous access device.
Although these devices proved to be a major overall advance since their introduction in the mid-Eighties, a variety of complications have been reported particularly if the devices are not cared for by experts. The complications include infection and various vascular clotting problems even paradoxical embolisation (Espiritu JD, Kleinhenz ME. Mayo Clin Proc 2000; 75:1100-1102. [PubMed]).
– Experience at CF centres shows that this present complication is not rare and most centres have experienced one or two cases.
Dr Susan Garwood (fig. 11) was, at this time, at the Department of Medicine, Medical University of South Carolina, Charleston, South Carolina.
Gibson RL, Retsch-Bogart GZ, Oermann C, Milla C, Pilewski J, Daines C, Ahrens R, Leon K, Cohen M, McNamara S, Callahan TL, Markus R, Burns JL. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol 2006; 41:656-665. [PubMed]

Fig.12 Ronald L Gibson
Aztreonam lysinate for inhalation (AI) is a novel monobactam formulation for chronic pulmonary Pseudomonas aeruginosa infections in CF. The activity of Al was retained against multiple CF isolates after nebulisation via eFlow nebuliser, and the activity was not inhibited by CF sputum. All 12 adult subjects and 11 of 12 adolescents tolerated the inhaled antibiotic.
These data were supportive of the continued development of aztreonam lysinate for treatment of patients with CF.Original aztreonam was not tolerated as an inhalation but the lysinate is suitable for nebulisation. This study also confirms that nebulisation does not denature the product.Phase III trials were completed by 2008 (Retsch-Bogart et al, 2008. below [PubMed] [PubMed]) and by 2009 the product licensed in Europe and Canada; and in the USA in 2010.
Dr Ronald L Gibson (Fig. 12) is at the Department of Pediatrics, University of Washington, Children’s Hospital and Regional Medical Center, Seattle, Washington 98105-0371, USA.
Gyi KM, Hodson ME, Yacoub MY. Pregnancy in cystic fibrosis lung transplant recipients: case series and review. J Cyst Fibros. 2006; 5:171-175. [PubMed]

Fig.13 Kim Gyi. Author’s photo
Report of 4 patients with CF and 6 further cases reviewed. There were nine live births and one therapeutic abortion. Although 5 babies were premature all were well at follow-up. Three mothers developed rejection during pregnancy and one had obliterative bronchiolitis and 3 died. The authors observe that although pregnancy in lung transplant recipients is feasible it is a risky undertaking.
Dr Kim Gyi (fig. 13) is a consultant respiratory physician at the Royal Brompton Hospital, London and involved with the care of the adults with cystic fibrosis and also various areas of clinical research.
Hafen GM, Ukoumunne OC, Robinson PJ. Pneumothorax in cystic fibrosis: a retrospective case series. Arch Dis Child 2006; 91:924-925. [PubMed]

Fig. 14. Gaudenz Hafen
ResearchGate
Pneumothorax is a known complication in cystic fibrosis, associated with poor outcome.Records of CF patients with pneumothorax at the Royal Children’s Hospital, Melbourne between 1990 and 2004 were reviewed, and the characteristics, sputum culture results, lung function, treatment, and outcome for the 11 patients who had pneumothoraces were described.
Pneumothorax is now rare in children with CF.
Gaudenz Hafen (Fig. 14) is at the Department of Respiratory Medicine, Royal Children’s Hospital, Melbourne, Victoria, Australia.
Hardin DS, Adams-Huet B, Brown D, Chatfield B, Dyson M, Ferkol T, Howenstine M, Prestidge C, Royce F, Rice J, Seilheimer DK, Steelman J, Shepherds R. Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial. J Clin Endocrin Metab 2006; 91:4925-9. [PubMed]

Fig. 15 Dana Hardin. Healthline
Sixty one prepubertal children were either treated with growth hormone or were controls. After one year, growth hormone treated children had significantly greater gain in height, weight, lean body mass, and bone mineral content. They had fewer hospitalizations and an improvement in CF quality of life but there was no difference in pulmonary function between groups. After cessation of GH treatment, there was a sustained effect for increased height and weight velocity, as well as accrual of bone mineral.
– Although there was a significant favourable effect for the growth hormone treatment there are few children who receive or indeed now require this treatment (also Hardin et al, 2001 above. [PubMed]).
Dr Dana Hardin (fig. 15) is at the Southwestern Medical School, University of Texas, Dallas, USA.
Hayes D Jr. Obstructive sleep apnea syndrome: a potential cause of lower airway obstruction in cystic fibrosis. Sleep Medicine 2006; 7:73-75.[PubMed]

Fig. 16 Don Hayes Jr
A six-year-old healthy female with cystic fibrosis (CF) and pancreatic sufficiency presented with cough, weight loss, and lung function decline. Further history suggested obstructive sleep apnea, and nocturnal polysomnography (NPSG) confirmed this. Adenotonsillectomy resulted in resolution of clinical symptoms with return of normal lung function.
– This case establishes that obstructive sleep apnea syndrome (OSAS) may be a potential cause of lower airway inflammation and resulting weight loss in the young CF population.It is useful to remember that increase in respiratory symptoms and signs in CF may be related to the upper respiratory tract which may be obstructed by the huge tonsils and adenoids. In such cases adenotonsillectomy may be followed by dramatic improvement even in young children not only in overnight oxygen saturations but also in weight increase and general wellbeing. A useful question to ask the parents is whether they can hear the child snoring in bed upstairs when they are downstairs – such children should be investigated!.
Dr Don Hayes Jr (fig. 16) was at this time at the Departments of Pediatrics and Medicine, University of Wisconsin Medical School, Madison. Subsequently Medical Director, Lung Transplant Program Cincinnati Children’s.
Kappler M, Kraxner A, Reinhardt D, Ganster B, Griese M, Lang T. Diagnostic and prognostic value of serum antibodies against Pseudomonas aeruginosa in cystic fibrosis. Thorax 2006; 61:684-688.
A representative cross sectional analysis of serum antibodies against three Pseudomonas antigens (alkaline protease, elastase, and exotoxin A) was performed in 183 patients with CF of mean age 16.7 years and FEV1 85.9% predicted. The results were correlated with microbiological results from the previous 2 years to calculate sensitivity, specificity, positive and negative predictive values. The following 2 years were assessed to determine prognostic predictive values.The authors concluded regular determination of serum antibodies may be useful in CF patients with negative or intermittent but not with positive P. aeruginosa status. A rise in antibody titres indicates probable infection and eradication treatment may be initiated even in the absence of microbiological detection of P. aeruginosa.
Dr M Kappler is at the Children’s University Hospital of the Ludwig-Maxmilians-University, Munich, Germany.
– A number of papers evaluating the use of Pseudomonas antibodies in CF have appeared regularly since the Seventies when Niels Hoiby showed by crossed immunoelectrophoresis that a rising antibody was a bad sign (Hoiby N, Axelsen NH. Identification and quantitation of precipitins against Pseudomonas aeruginosa in patients with cystic fibrosis by means of crossed immunoelectrophoresis with intermediate gel. Acta Path Microbiol Scand 1973; 81:298-308. [PubMed])
In Leeds, I was influenced by Hoiby’s antibody work, in the mid-Eighties, we evaluated their use (Brett MM, Ghoneim ATM, Littlewood JM. Serum antibodies to Pseudomonas aeruginosa in cystic fibrosis. Arch Dis Child 1986; 61:1114-1120.[PubMed]). In a series of studies by Dr Moira Brett we found them to be very useful in patient management. Basically the absence of antibodies was reassuring that Pseudomonas was not present in the tissues of the airways and a rising titre in chronically infected patients indicated the need for more aggressive treatment. Raised antibodies and negative cultures prompted bronchosopy for BAL.
Kalnins D, Ellis L, Corey M, Pencharz PB, Stewart C, Tullis E, Durie PR. Enteric-coated pancreatic enzyme with bicarbonate is equal to standard enteric-coated enzyme in treating malabsorption in cystic fibrosis. J Pediatr Gastroenterol Nutr 2006; 42:256-261. [PubMed]

Fig 17. Diana Kalnins
To compare the efficacy of an enteric-coated buffered pancreatic enzyme containing 1.5 mEq of bicarbonate per capsule with a conventional enteric-coated enzyme capsule in cystic fibrosis patients with signs or symptoms of moderate to severe malabsorption. The authors found that In the doses used, nutrient absorption of patients taking the buffered preparation offered no advantage over a conventional preparation.The addition of bicarbonate or acid suppressing drugs was recommended to protect the active enzymes from gastric acid before the acid resistant enzymes became available in the early Eighties. With the introduction of the acid resistant microspheres (e.g. Pancrease and Creon) acid suppression was required infrequently and only recommended if malabsorption was difficult to control even with doses equivalent to 10,000IU lipase/kg/day. Following the recognition of fibrosing colonopathy related to very high doses of some brands of pancreatic enzymes, there was a renewal of interest in acid suppression as a means of reducing the dose of enzymes.
Diana Kalnins (fig.17) is Academic and Clinical Specialist Dietitian, Respiratory Medicine Manager, Clinical Dietetics at the Hospital for Sick Children in Toronto, Ontario. In addition to many research publications on CF she has co-authored several books on nutrition, including The Hospital for Sick Children’s Better Food for Kids and Better Baby Food, which won The International Cookbook Revue Award (2001).
Katznelson D. On the complexity of the pulmonary microbiology in cystic fibrosis: thoughts of a clinician. Isr Med Assoc J 2006; 8:49-52. [PubMed]

Fig. 18 Daniel Katznelson Author’s photo
The pulmonary microbiology is a dominant element in cystic fibrosis and the main cause of death. Contemporary consensus accords an exclusive role in this to a single microorganism, Pseudomonas aeruginosa. The evidence convincingly shows that the microbiology consists of a multiplicity of species living in perpetual interaction and in a variety of forms–planktonic, sessile, anaerobic–and in organized communities as microcosms, biofilms and ecosystem. This compound microbiology, the essence of the pulmonary disease, is of necessity exposed to constant influence both from without (the air) and within (via the blood), leading to a perpetual state of flux with consequent impact on the clinical course. It is perhaps significant that to date, most or all microbiologic studies were probably conducted, classically, with inert instruments (glass? plastic?), whereas in real life the CF microbiology lives in “test-tubes” of live mucosa with which it maintains a permanent “cross-talk.” The difference to microbial life between these two media may well be very important. It therefore justifies study and may be far-reaching in its effect. There is persuasive argument to strive for a novel holistic view of the totality of the complex microbiology of CF, and to initiate fresh concepts, strategies and methods. These are thoughts of Israel’s pioneer in CF care and research,
Professor Daniel Katznelson (fig.18) of the Department of Pediatrics, Sheba Medical Center, Tel Hashomer, Israel, has been involved with many aspects of CF care and research since the Sixties.
Lipecka J, Norez C, Bensalem N, Baudouin-Legros M, Planelles G, Becq F, Edelman A, Davezac N. Rescue of DeltaF508-CFTR (cystic fibrosis transmembrane conductance regulator) by curcumin: involvement of the keratin 18 network. J Pharmacol Exp Ther 2006; 317:500-505. [PubMed]

Fig. 19 Joanna Lipecka
LinkedIn
Recently, curcumin was shown to rescue DeltaF508-CFTR localization and function in mice (Egan et al. Science 2004; 304:600-602 above). In previous work from the present authors, the keratin 18 (K18) network was implicated in DeltaF508-CFTR trafficking.
Here, the authors hypothesize that curcumin could restore a functional DeltaF508-CFTR to the plasma membrane acting via the K18 network. They proposed K18 as a new therapeutic target and curcumin, and/or its analogs, might be considered as potential therapeutic agents for cystic fibrosis.
This study provides an explanation for the effect of curcumin but unfortunately the curcumin effect reported by Egan et al (2004 above) could not be reproduced by others although interest continued in the potential role of curcumin (Colas J et al. Disruption of cytokeratin-8 interaction with F508del-CFTR corrects its functional defect. Hum Med Genet 2012; 21:623-634. [PubMed]).
Joanna Lipecka (fig.19) is at the Institut National de la Sante et de La Recherche Medicale U467, Université René Descartes Paris 5 Faculté de Meédicine Paris 5 Paris France
Lloyd-Still JD, Powers CA, Hoffman DR, Boyd-Trull K, Lester LA, Benisek DC, Arterburn LM. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition 2006; 22:36-46.[PubMed]

Fig 20. John Lloyd-Still. Doctor.com
Several studies have reported omega-3 and omega-6 fatty acid imbalances in patients with cystic fibrosis. Whether these imbalances contribute to, or are manifestations of, the pathophysiology of CF is unknown. This study by John Lloyd-Still and colleagues from Chicago was to determine bioavailability, tissue accretion, and safety of a large dose of an algal source of docosahexaenoic acid (DHA) triacylglycerol and to observe the effects on lung function in patients with CF. Twenty subjects with CF (8 to 20 yrs of age) were randomly assigned to receive algal oil providing 50 mg of DHA per kilogram per day (1 to 4.2 g of DHA per subject per day) or placebo for 6 months. The authors found that algal DHA triacylglycerol oil is readily absorbed well tolerated, and increases blood and tissue DHA levels in patients with CF. No adverse developments were associated with this large dose of DHA oil.
The authors concluded that larger studies of longer duration are needed to determine whether DHA supplementation results in any clinically significant benefits in patients with CF.
Subsequently a report from Belgium failed to show any clinical improvement after a year’s supplementation with a DHA rich algal oil (Van Biervliet S et al. Prostaglandins Leukot Essent Fatty Acids 2008; 78:109-115. [PubMed]).
Dr John Lloyd Still (fig.20 ) is one of the leading figures in CF care and research in N. America and has been involved in CF and paediatric gastroenterology for many years since he qualified at Guys Hospital in London in 1960. After qualifying he worked in London in paediatrics and eventually moved to Boston where he worked with Harry Shwachman. He edited a major textbook on CF in 1983 to which most of the leading authorities on CF in North America at the time contributed (Textbook of Cystic Fibrosis. Lloyd-Still J D. John Wright PSG Inc. 1983). He is particularly interested in, and has published widely on, the gastroenterological and nutritional aspects of CF.
Littlewood JM, Wolfe SP, Conway SP. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol 2006; 41:35-49. [PubMed]

Fig 21. Sue Wolfe & Jim Littlewood at ECFS Valencia
A detailed review of the present management of malabsorption based on some 25 years experience at the Regional Paediatric CF Centre in Leeds. We have been very fortunate in Leeds in having a number of outstanding paediatric dietitians since Anita MacDonald (now Chief Dietitian at Birmingham Children’s Hospital) played a major part in building up the Regional Paediatric CF Unit at St James’s during the Eighties.
Sue Wolfe,(fig.21) the chief paediatric dietitian to 2017, and Alison Morton the recently retired chief dietitian on the adult CF unit, at St James’s Leeds have made a major contributions to the nutritional aspects of CF both locally and internationally and now have vast practical and research experience.

Fig. 22 Jerry Kelleher & Mike Walters
Much of our nutritional and gastrointestinal work was in collaboration with members of Professor Monty Losowsky’s University Department of Medicine in St James’s where there was a very productive collaboration between the adult gastroenterologists and our CF team. Particular mention going to the late Dr Jerry Kelleher the chief biochemist and his colleague Mr Mike Walters.
Lebecque P, Leal T, Zylberberg K, Reychler G, Bossuyt X, Godding V. Towards zero prevalence of chronic aeruginosa infection in children with cystic fibrosis. J Cyst Fibros 2006; 5:237- 44. [PubMed]

Fig. 23 Patrick Lebecque
chi.be
Prevalence of chronic P. aeruginosa infection in one of the seven Belgian CF centres was very low and only half the national level; overall 19.8% for all the children and adults but only 2.8% in patients below 18 years. This was attributed to cohorting patients according to their microbiological status and particularly to the widespread and frequent use of inhaled antibiotics over the previous 15 years; also patients were seen by the same paediatric pulmonologist
This is an impressive report showing a 2.8% prevalence of chronic P. aeruginosa infection in children at this Belgian CF clinic – a prevalence in stark contrast to many published results on the incidence reported in both the US and UK patient registries. In addition to an aggressive approach to eradication of early P. aeruginosa infection, and also being seen by the same paediatric pulmonologist, will have made an important contribution to these excellent good results.
Dr Patrick Lebecque (fig. 23) is Paediatric Pulmonologist at Centre de Reference pour la Mucoviscidose, Clinques St Luc, Brussels. He is active and publishes widely not only in CF research but also in clinical care as evidenced by these excellent results. Presumably he was “the same pulmonologist” who contributed to these excellent results.
Li N, Pei P, Bu DF, He B, Wang GF. A novel CFTR mutation found in a Chinese patient with cystic fibrosis. Chin Med J 2006; 119:103-109. [PubMed]
A Chinese girl of 16 years old was diagnosed as having CF at the age of 14 years. A heterozygous novel missense mutation of 699 C –> A, which results in the amino acid change of N189K, was identified in exon 5. In addition, a heterozygous 3821 – 3823 delT mutation in exon 19 was found in CFTR.Cystic fibrosis is very rare on Chinese people; the twenty patients reported from 1974 to 2004 were also reviewed in this paper. DelF508 mutation was not found in any of the nine cases whose CFTR mutations were analyzed and suggests that the genotype of Chinese CF may be different from those in Europe and N America.
Lording A, McGraw J, Dalton A, Beal G, Everard M, Taylor CJ. Pulmonary infection in mild variant cystic fibrosis: implications for care. J Cyst Fibros 2006; 5:101-104. [PubMed]

Fig. 24 Chris Taylor
Author’s photo
Few reports document the condition of the airway in infants and young children with apparent so-called “mild” disease. A retrospective cohort study was carried out comparing frequency of bacterial isolates and clinical outcomes in eleven compound heterozygotes for DeltaF508 and a second mild mutation, mainly R117H, with a matched group of DeltaF508 homozygotes.Staphylococcus aureus was isolated in 8 of the 11 patients with mild variant disease and Pseudomonas aeruginosa found in 7 (64%), although the frequency of positive cultures was significantly less (2.8/year) than the DeltaF508 homozygotes (6.1/year). Shwachman scores were significantly higher in patients with mild mutations (94(74-92) vs. 88 (77-91)); there was also a small but significant difference in chest radiograph (Chrispin-Norman) scores although little difference in lung function.
– This is a timely paper from Sheffield UK stressing that most patients with so-called “mild” variant CF will nonetheless have bacterial isolates from their airway cultures requiring antibiotic therapy three or four times a year. Infection with both S. aureus and P. aeruginosa is common in these patients and they are slowly deteriorating with respect to respiratory function. In the UK, we believe that anti-staphylococcal prophylaxis at least for the first three years is indicated. Also, it is vitally important these so-called “mild patients” are followed carefully by a team experienced in CF care and treated vigorously to prevent their changing into “severe cases”. They deserve just as careful treatment and follow-up as those with more obvious symptoms and signs – unfortunately, they may be regarded as “not bad enough to be referred to a CF Centre” by the local physician.
Prof. Chris Taylor of Sheffield (fig 24) is one of the UK’s leading CF experts both clinically and in terms of scientific gastrointestinal research.
Lucidi V, Tozzi AE, Bella S, Turchetta A. A pilot trial on safety and efficacy of erythrocyte-mediated steroid treatment in CF patients. BMC Pediatrics 2006;. 6:17. [PubMed]
A novel strategy for delivering low doses of steroids for long periods through the infusion of autologous erythrocytes loaded with dexamethasone. A recent study suggested the feasibility of therapy with low doses of corticosteroids delivered through engineered erythrocytes in CF patients. This study presents a further analysis of safety and efficacy of this therapy. Nine patients in the experimental group received the treatment once a month for a period of 24 month.Patients did not develop diabetes, cataract, or hypertension, or other typical side effects of steroid treatment during the follow up period. There was a constant improvement of FEV1 in patients undergoing the experimental treatment compared to a gradual decrease of the same parameter in the standard therapy group (P = 0.04). The average of clinic and radiological indexes did not vary. The number of infective relapses that have required antibiotic intravenous therapy was not different in the two groups, although the average of these episodes was slightly higher in the experimental therapy group.
The authors concluded intraerythrocyte corticosteroid treatment may stabilize the respiratory function in CF patients but is often considered too invasive by patients. The results obtained by their study may help planning an experimental, controlled, randomised study. A novel method for administering anti-inflammatory treatment with dexamethasone to people with CF.
– However, it is no surprise that there there were no further studies on this involved technique up to 2023.
Dr V Lucidi is at the Ospedale Bambino Gesù, Department of Pediatrics, Cystic Fibrosis Unit, Rome, Italy.
Madge S. Growing up and growing older with cystic fibrosis. J R Soc Med 2006; 99 (Suppl):23-26. [PubMed]

Fig 25. Sue Madge
A useful review of the problems associated with transition and adulthood dealing with most of the major issues that cause concern from transition to end of life issues. The article is based on extensive experience of the author.
Dr Sue Madge (fig. 25), has extensive experience as a consultant CF Nurse Specialist first at Great Ormond Street Hospital and then at the Royal Brompton in London. Sue has also been an advisor on many aspects of CF care to the CF Trust and other European organisations. She was awarded the John Panchaud Medal of the CF Trust for her work with cystic fibrosis.
Martin SL, Downey D, Bilton D, Keogan MT, Edgar J, Elborn JS. Recombinant AAT Study Team. Safety and efficacy of recombinant alpha (1)-antitrypsin therapy in cystic fibrosis. Pediatr Pulmonol 2006; 41:177-183. [PubMed]

Fig. 26 Lorraine Martin
qub.ac.uk
A previous study had been performed with plasma derived inhibitor (McElvaney et al, 1991 above. [PubMed]). In this present study recombinant human ATT was used in a Phase II trial of rAAT at various dose levels. This drug was safe but, disappointingly, had little effect on neutrophil elastase activity and other markers of inflammation. As a result, there was no further work in CF from this group.
However, later a similar trial from Germany (Griese, M. et al, Eur Respir J 2007; 29:240-50. [PubMed]) did show some reduction of inflammatory markers although no change of respiratory function.These authors suggested that the clear reduction of airway inflammation after ATT treatment may precede pulmonary structural changes; also the ATT deposition region, either bronchial or peripheral, may play a minor role for ATT inhalation in patients with cystic fibrosis. So it is likely there may be further developments in relation to CF.
Professor Lorraine Martin (fig.26) is at the School of Pharmacy, Queen’s University Belfast, Belfast, Northern Ireland, UK At the outset of her career, she played a pivotal role in a joint programme between Queen’s University Belfast and the biotechnology company behind Dolly the cloned sheep, PPL Therapeutics plc, Edinburgh and was closely involved in the Phase II clinical trials of a transgenic human protein, known as alpha1 antirypsin in cystic fibrosis (CF).
Montgomery GS, Sagel SD, Taylor AL, Abman SH. Effects of sildenafil on pulmonary hypertension and exercise tolerance in severe cystic fibrosis-related lung disease. Pediatr Pulmonol 2006; 41:383-385. [PubMed]

Fig. 27 Gregory S Montgomery
medicine.iu.edu
Cystic fibrosis (CF) patients with advanced lung disease are at risk for developing pulmonary vascular disease and pulmonary hypertension, characterized by progressive exercise intolerance beyond the exercise-limiting effects of airways disease in CF.
The authors report on a patient with severe CF lung disease who experienced clinically significant improvements in exercise tolerance and pulmonary hypertension without changing lung function during sildenafil therapy.
Sildenafil has been reported to have a favourable effect on the DF508 mutation although no clinical trials had been reported by 2013, although there is work in mice (Lubamba B et al, 2011.[PubMed]) and a review of PDE5 inhibitors as treatment for CF in 2012 (Noel S et al. 2012. [PubMed]). A recent report indicates sildenafil treatment 4 wk of sildenafil treatment improves the capacity of the skeletal muscle to use O2 more efficiently during exercise.(Pula Rodridguez-Miguelez et al, 2021 [pubmed.ncbi.nlm.nih.gov/33105385/]
Dr Gregory S Montgomery (fig. 27) is Associate Professor of Clinical Pediatrics Division of Pediatric Pulmonology, Department of Pediatrics, Indiana University Medical Center, Indianapolis, Indiana, USA.
McLaughlin AM, Crotty TB, Egan JJ, Watson AJ, Gallagher CG. Amyloidosis in cystic fibrosis: a case series. J Cyst Fibros 2006; 5:59-61.[PubMed]

Fig. 28 Anne Marie McLaughlin
ResearchGate
As the life expectancy of patients with cystic fibrosis increases, unusual complications including amyloidosis are increasingly recognised. The authors report three cases of amyloidosis including a unique case presenting with a hemorrhagic and thrombotic diathesis. This complication of amyloidosis has never been reported in cystic fibrosis.
There are sporadic reports of amyloidosis in people with CF since the Seventies. Considering the prolonged severe sepsis present in many patients the number of reports are relatively infrequent. See Topics for Castile R, et al. Amyloidosis as a complication of cystic fibrosis. Am J Dis Child 1985; 139:728-732.[PubMed]
Ann Marie McLaughlin (fig.28) was at the Department of Medicine and Therapeutics, St Vincent’s University Hospital , Dublin. Subsequently she was appointed consultant at St James’s University Hospital Leeds.
McKone EF, Goss CH, Aitkin ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest 2006; 130:1441-1447. [PubMed]

Fig 29. Ed McKone DublinLive
Data from CF Foundation registry on 15,651 patients was analysed and showed that the high risk CFTR genotype carried a twofold increased risk of death compared to those with low risk genotype. Of patients who died, the high risk median age of death was 24.2 years and low risk 37.6 years. Although reassurance can be provided for patients in the low risk group there is substantial phenotypic variability.The outlook for people with CF is mainly determined by the treatment they receive although a minority do have mutations which are associated with milder disease; these are often associated with pancreatic sufficiency and a much later presentation and diagnosis.
Dr Ed McKone (fig.29) is a consultant respiratory physician at St Vincent’s Hospital National Adult CF Centre in Dublin. He is Director of the European Patient Registry.
Melzi ML, Kelly DA, Colombo C, Jara P, Manzanares J, Colledan M, Strazzabosco M, DeLorenzo P, Valsecchi MG, Adam R, Gridelli B, Assael BM, EGSLTCF, European Liver Transplant Association (ELTA), European Cystic Fibrosis Society (ECFS). Liver transplant in cystic fibrosis: a poll among European centers. A study from the European Liver Transplant Registry. Transplant International 2006; 19:726-731. [PubMed]
A report of 57 CF patients, many of whom were in their teens, who had liver transplants. Post-transplant survival was high and poor respiratory function was the main risk factor for early death; in the short-term, respiratory function significantly improved in most patients. Transplantation was considered to be the appropriate treatment for patients with advanced CF-related liver disease and preserved pulmonary function and an FEV1 over 50% predicted.Further general European experience of liver transplantation in people with CF confirming the good results since the first report of Mieles LA et al. (1989 above) [PubMed]

Fig. 30 Diedre Kelly CBE
wmlieutenancy.org
Prof. Deirdre Kelly (fig.30) is a consultant paediatric hepatologist at the Liver Unit of Birmingham Children’s Hospital and Reader in Paediatric Hepatology at the University of Birmingham. Deirdre is subsequently Professor of Paediatric Hepatology at the University of Birmingham, Consultant Paediatric Hepatologist and Founding Director of the Liver Unit for Birmingham Children’s Hospital NHS Foundation Trust. She set up the Liver Unit at Birmingham Children’s Hospital which provides a national and international service for children with liver failure and undergoing liver transplantation, transforming survival and outcome for these children. She runs an active research programme focusing on viral hepatitis in children, molecular genetics of inherited liver disease, quality and outcome of life following liver and/or intestinal transplantation. The Unit has been very successful and is one of the busiest paediatric liver transplant programmes with survival rates of over 90%.
Dr M L Melzi is at the Liver Transplant Unit, Ospedali Riuniti, Bergamo, Italy
Mulheran M, Hyman-Taylor P, Tan KH, Lewis S, Stableforth D, Knox A, Smyth A. Absence of cochleotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times-daily tobramycin in cystic fibrosis patients. Antimicrob Agents Ch 2006; 50:2293-9. [PubMed]

Fig 31 Michael Mulheran
ResearchGate
The authors undertook assessment of hearing in patients with cystic fibrosis who were taking part in a large randomized controlled trial of once- versus three-times-daily tobramycin for pulmonary exacerbations of cystic fibrosis (the TOPIC study). Complete pre- and post treatment standard audiological data were obtained for 168/219 patients. They found no significant differences in hearing thresholds when they were assessed at the baseline, at the end of treatment, and at follow-up 6 to 8 weeks later were compared. In addition, no significant differences in hearing thresholds were detected between treatment regimens. Similar results were obtained for the subset of 63/168 patients who underwent high-frequency audiometry.The authors concluded that for a single 14-day course of tobramycin treatment in patients with cystic fibrosis with no preexisting auditory deficit, no measurable effect on hearing was apparent with either once- or three-times-daily treatment. The suggested that estimation of the cumulative cochleotoxic risk in cystic fibrosis patients due to repeated aminoglycoside therapy, as evidenced by the patients excluded from this study due to hearing loss, also requires further characterization.
Michael Mulheran (fig.31) is at the MRC Centre for the Study of Mechanisms of Human Toxicity, Neurotoxicology, University of Leicester, Leicester LE1 9HN, United Kingdom
Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem 2006; 281:27942-9. [PubMed]

Fig 32. Ray Frizzell
This study, using primary human airway epithelial cells, shows (i) protease inhibitors are present in the airway surface liquid (ASL) and prevent the activation of near-silent ENaC, (ii) when the ASL volume is increased, the endogenous protease inhibitors become diluted, allowing for proteolytic activation of near-silent channels, and (iii) in CF, the normally present near-silent pool of ENaC is constitutively active and the alpha subunit undergoes increased proteolytic processing.These findings indicate that the ASL volume normally modulates the activity of ENaC by modification of the serine protease-protease inhibitor balance and that alterations in this balance contribute to the excessive sodium absorption in cystic fibrosis. A neat explanation for the control of the ENaC channels.
Professor Raymond Frizzell (fig. 32) is Professor and Director of the Cystic Fibrosis Research Center Department of Cell Biology and Physiology. University of Pittsburgh School of Medicine. He and his group are interested in the mechanisms of chloride and sodium transport across absorptive and secretory cell surfaces.
Obideen K, Wehbi M, Hoteit M, Cai Q. Nocturnal hydration: an effective modality to reduce recurrent abdominal pain and recurrent pancreatitis in patients with adult-onset cystic fibrosis. Dig Dis & Sci 2006; 51:1744-1748. [PubMed]

Fig. 33 Kamil Obideen
United Digestive
The proportion of older people with CF who have recurrent troublesome abdominal symptoms is as high as 20-30% in some clinics. In this study patients were encouraged to drink plenty of water through the night and assessed with regard to abdominal symptoms for 3 months before and after starting this regimen. The frequency and severity of pain was reduced as was the medication required, emergency room visits and hospitalisations and even episodes of acute pancreatitis.
This is a simple measure which would be likely to reduce the pain which, in our experience, is commonly due to constipation and faecal accumulation in the lower ileum and colon even though the patient does not complain of constipation (i.e. defined as the passage of infrequent hard stools).
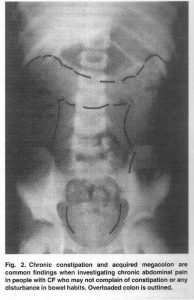
Fig 34. Please see text for details
This child (fig. 34) complained of troublesome recurrent central abdominal pain; but neither she nor her parents complained of any disturbance in her bowel habit. However, her abdominal pain resolved completely when she was treated with laxatives (Littlewood et al, Pediatr Pulmonol 2006; 41:35-49.[PubMed]).
So this suggestion of increasing fluid intake is simple, cheap and potentially very useful measure to reduce the incidence of abdominal pain in adults with CF. An extremely economical, and apparently effective, way of improving quality of life. An additional reason for persisting abdominal symptoms could be related to the presence of inflammation of the bowel which has been shown in a number of recent studies by breath tests, inflammatory markers in the stools, endoscopic means and most recently by capsule visualisation.
Dr Kamil Obideen (fig.33) is in the Division of Digestive Diseases, Emory University School of Medicine, 1365 Clifton Road, Suite B 1262, Atlanta,
Phaff SJ, Tiddens HA, Verbrugh HA, Ott A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J Antimicrob Chemother 2006; 57:741-746. [PubMed]
The purpose of this study was to determine the association between long-term use of azithromycin, now taken by many patients with CF, and change over time in macrolide susceptibility of Staphylococcus aureus and Haemophilus spp. The authors found that erythromycin resistance in S. aureus increased from 6.9 to 53.8% and clarithromycin resistance in Haemophilus spp. from 3.7 to 37.5%. Resistance but also isolation rates were strongly related to azithromycin use.
So over a 4 year period, azithromycin maintenance therapy in the CF population was associated with an significant increase in macrolide resistance in S. aureus and Haemophilus spp. which was not unexpected.
– In 2021 Azithromycin is a recommended therapy and taken by 55.6% gof people with CF age of 6 years and over (2021 CFF Patient Registry)
Sonja P Pfaff is in the Department of Pediatric Pulmonology and Allergology, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands.
Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL. CALICO Trial Collaborative Group. Oral protein energy supplements for children with cystic fibrosis: CALICO multicentre randomised controlled trial. BMJ 2006; 332(7542):632-636. [PubMed]

Fig. 35 Vanessa Poustie LinkedIn
In 102 children with cystic fibrosis, aged between 2 and 15 years, who were moderately malnourished, the effects of oral protein energy supplements in addition to their usual dietary advice were compared with dietary advice alone, for 12 months.
The authors concluded that the long term use of oral protein energy supplements did not result in a significantly better improvement in nutritional status or other clinical features and so oral protein energy supplements should not be regarded as an essential part of the management of children with CF.
This multi centre trial was supported by the UK CF Trust. At the time over 50% of children with CF in the UK were taking regular (expensive) proprietary dietary supplements with only sparse evidence of their efficacy. Although this trial showed that dietary advice to increase the intake of normal food was of equivalent value, the supplements were still widely used as one way of dealing with the eating problems so common in small children with CF and in improving energy intake in adults (White et al, J Cyst Fibros 2004; 3:1-7. [PubMed]).
Dr. Vanessa Poustie (fig. 35) is Assistant Director Medicines for Children Research Network, University of Liverpool, UK.
K.Prasad SA, Balfour-Lynn IM, Carr SB, Madge SL. A comparison of prevalence of urinary incontinence in girls with cystic fibrosis, asthma and healthy controls. Pediatr Pulmonol 2006; 41:1065-1068. [PubMed]

Fig 36. Ammani Prasad
Another study on urinary incontinence – this time on younger patients. In recent years the physiotherapists have taken an increasing interest in bladder dysfunction in CF. Girls with CF aged 11 to 17 years were studied and urinary incontinence was reported by 17/51 (33%) girls, compared with only 4/25 (16%) of those with asthma and 2/27 (7%) healthy controls. The problem was associated with increasing severity of lung disease. (also described in adults with CF by Cornacchia et al, 2001 above[PubMed]; Orr A et al. BMJ 2001; 322:152.[PubMed]; and treatment discussed by McVean RJ et al. J Cyst Fibros 2003; 2:171-176.[PubMed]).
Armmani Prasad (fig. 36) is a senior Physiotherapist and Manager of the CF Unit at Great Ormond Street Hospital for Children, London and one the leading CF physiotherapists both in the UK and internationally. She has written extensively on CF and also been an invited speaker at many conferences both in the UK and abroad and regularly advises the CF Trust on matters relating to CF care.
Proesmans M, Balinska-Miskiewicz W, Dupont L, Bossuyt X, Verhaegen J, Hoiby N, de Boeck K. Evaluating the “Leeds criteria” for Pseudomonas aeruginosa infection in a cystic fibrosis centre. Eur Respir J 2006; 27:937-943.[PubMed]

Fig. 37 Marijke Proesmans
Four separate categories of chronic Pseudomonas aeruginosa (Pa) infection in children with cystic fibrosis have been previously defined, based on airway cultures taken over the previous year. The aim of the present study was to evaluate this definition in the current authors’ paediatric and adult CF clinic using clinical, immunological and lung function parameters. During follow-up, out of 193 patients, 55 (34%) CF patients had never been infected with Pa, 27 (17%) were free of Pa, 29 (18%) were intermittently infected and 51 (31%) were chronically infected. Disease severity markers, such as lung function, were significantly worse in the chronic group, especially in the paediatric population. Differences in adult patients were smaller and no longer significant. Pa antibodies differed strongly between the groups, and were very high (mean+/-sd 55.4+/-5.5) and highly statistically significant from all other groups in the chronic group. They were low and different from all other groups in the never group (1.8+/-0.6). Pa antibodies did not differ between the free of Pa and the intermittent group.In conclusion, the current authors confirmed an agreement between Pseudomonas aeruginosa status according to the new Leeds definition and clinical status, as well as with the level of Pseudomonas aeruginosa antibodies. Although these authors agreed with the Leeds criteria for evaluating Pseudomonas infection, there were still considerable differences in the criteria used for chronic PA infection in subsequent publications.
Professor Marijke Proesmans (fig.37) is Consultant in Paediatric Pulmonology at the University Hospital Leuven, Belgium. Marijke Proesmans graduated as a medical doctor at the University of Leuven, Belgium and performed her paediatric training at the same institution. After obtaining a PhD, she chose for further clinical training in paediatric pulmonology and respiratory revalidation and worked at the respiratory department of Great Ormond Street Children’s Hospital in London. For several years she has been a consultant in the paediatric pulmonology department of the University Hospital of Leuven which, includes a large CF referral centre. She is also a lecturer at the medical faculty of the University of Leuven. She has published many studies relating to chest diseases in children and particularly cystic fibrosis.
Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemo2006; 57:306-311. [PubMed]

Fig. 38. Felix Ratjen
Inhaled colistin is commonly used in patients with cystic fibrosis (CF), but only limited data are available to define its pharmacokinetic profile. We performed a multicentre study in 30 CF patients to assess sputum, serum and urine concentrations after a single dose of 2 million units of colistin administered by inhalation. In a subgroup of patients we also compared the efficacy of two different nebulizers for administration of inhaled colistin. Serum concentrations of colistin reached their maximum 1.5 h after inhalation and decreased thereafter. Serum concentrations were well below those previously reported for systemic application in all patients. A mean 4.3+/-1.3% of the inhaled dose was detected in urine. Elimination characteristics did not differ significantly from those previously reported for systemic application. A positive correlation was found between forced expiratory volume in 1 s (FEV1) in per cent predicted and both AUC and maximal colistin concentrations in serum (Cmax). Maximum sputum concentrations were at least 10 times higher than the MIC breakpoint for Pseudomonas aeruginosa proposed by the British Society for Antimicrobial Chemotherapy. Although sputum drug concentrations decreased after a peak at 1 h, the mean colistin concentrations were still above 4 mg/L after 12 h. No differences were seen in polymyxin E sputum concentrations, for CF patients between the two nebulizer systems.
– The authors concluded the low systemic and high local concentrations of colistin supported the use of inhaled colistin in CF patients infected with P. aeruginosa.This was reassuring as inhaled colistin had been increasingly used over the past 20 years since the first report of its use in eradicating early Pseudomonas infection in CF (Littlewood et al, Nebulised colomycin for early Pseudomonas colonisation in cystic fibrosis. Lancet 1985; I: 865. [PubMed]). No pharmacokinetic studies were done before we gave inhaled colomycin in the early Eighties to see if it would clear early Pseudomonas infection so the results of the present study were reassuring!
Rao DS, Infeld MD, Stern RC, Chelimsky TC. Cough-induced hemiplegic migraine with impaired consciousness in cystic fibrosis. Pediatr Pulmonol 2006; 41:171-176.pubmed.ncbi.nlm.nih.gov/16372353/

Fig. 39 Dinesh Rao
LinkedIn
The coughing paroxysms of patients with cystic fibrosis may occasion neurological symptoms. Although cough syncope is well-known, and is associated with headache and paralysis, a migrainous mechanism has not been reported. The authors reviewed the medical records, autonomic testing results, and responses to treatment in two cystic fibrosis patients with similar presentations of cough-induced impairment of consciousness followed by headache and paralysis.
A 24-year-old woman and an unrelated 38-year-old man, both with cystic fibrosis, developed post-tussive neurologic deficits. Both patients reported infrequent dramatic spells, always preceded by major hemoptysis, and associated with left-sided paralysis, transient blindness, nausea, and severe pulsating headaches. Autonomic testing demonstrated only postural tachycardia and a near-vasodepressor episode in the woman, and mild, generalized sympathetic dysfunction in the man. Treatment for presumptive migraine with aura with verapamil nearly eradicated symptoms in both patients. Discontinuation of verapamil in the woman was associated with symptom recurrence and a stroke, with significant persistent residual left hemiparesis.In conclusion, cough-induced neurologic deficits were previously reported with cystic fibrosis, without clear understanding of the mechanism of impairment of consciousness. Based on the hemiparesis, nausea, and throbbing headache, which repeatedly followed the events in both patients, and based on the response to verapamil, the authors hypothesize a migrainous mechanism in both of our patients. They suggest the pathophysiology that links the hemoptysis to the spells deserves further investigation.
– Another type of neurological complication resulting from pulmonary problems in people with CF.
Dinesh S Rao (fig 39) is in the Department of Neurology, Case Western Reserve University School of Medicine and University Hospitals of Cleveland, Cleveland, Ohio
Rønne Hansen C, Pressler T, Høiby N, Gormsen M. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J Cyst Fibros 2006; 5:245-251. [PubMed]

Fig. 40 Christine Hansen
ResearchGate
In cystic fibrosis (CF), chronic infection of the airways with Achromobacter xylosoxidans have become more frequent. The pathogenic role of this is yet unclear. A retrospective case-control study of all patients chronically infected with A. xylosoxidans for at least 3 years. 15 patients (6 males) with chronic A. xylosoxidans infection were matched by age, FEV(1) and body mass index z-score to 15 controls (7 males) at the time of establishment of chronic infection. Eight patients harboured a common A. xylosoxidans strain, indicating either cross-infection or a common source. The authors conclude that A. xylosoxidans may lead to a decline in lung function in a subgroup of chronically infected CF patients characterised by a rapid increase in specific precipitating antibodies. Cross-infection may possibly occur.A useful review from the Copenhagen CF Centre of a relatively new problem in people with CF.
Dr Christine Hansen (fig.40) at the Copenhagen Cystic Fibrosis Center, Departments of Paediatrics, Clinical Microbiology and Radiology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark. Subsequently at Paediatric Department, Skane University Hospital, Sweden
Robberecht E, Van Biervliet S, Vanrentergem K, Kerremans I. Outcome of total splenectomy with port systemic shunt for massive splenomegaly and variceal bleeding in cystic fibrosis. Pediatr Surg 2006; 41:1561-5.[PubMed]

Fig. 41 Eddy Robberecht
Artimino 2001
Six patients with CF (median age 14 years) underwent splenectomy with a splenorenal shunt operation – 3 for massive splenomegaly and three to control variceal bleeding after several sessions of sclerotherapy. Lung function remained stable, and there was an overall reduction of respiratory tract infections. The youngest patient, however, died of overwhelming septicaemia during treatment with steroids.
Sometimes the spleen is so large in people with CF that the size is a physical handicap causing respiratory embarrassment and considerable abdominal distension in addition to the systemic effects of hypersplenism. A boy with CF of 15 years in Leeds had a spleen weighing over 3 kg removed by the late Professor Geoff Giles with great improvement in his respiratory function and general health (Gilbert J et al. Splenectomy for massive splenomegaly in cystic fibrosis with improvement in pulmonary status. Scand J Gastroenterol 1987; 23 (Suppl 143):177).
Dr Eddy Robberecht (fig.41) is a senior paediatrician from Ghent University Dept of Pediatrics and Medical Genetics and has been involved in patient care and research particularly in the areas of nutrition, osteoporosis and liver involvement in which he has published extensively.
Saker A, Benachi A, Bonnefont JP, Munnich A, Dumez Y, Lacour B, Paterlini-Brechot P. Genetic characterisation of circulating fetal cells allows non-invasive prenatal diagnosis of cystic fibrosis. Prenatal Diagnosis 2006; 26:906-916. [PubMed]
The purpose of this study from Paris was to develop a molecular method to characterise both paternal and maternal CFTR alleles in DNA from circulating fetal cells (CFCs) isolated by ISET (isolation by size of epithelial tumour/trophoblastic cells). This protocol was validated in 12 pregnant women, at 11 to 13 weeks of gestation, whose offspring had a 1 in 4 risk of CF. Results showed that one fetus was affected, seven were heterozygous carriers of a CFTR mutation, and four were healthy homozygotes. These findings were consistent with those obtained by chorionic villus sampling (CVS).
This test affords a reliable method prenatal diagnosis for high risk couples and avoids the risks associated iatrogenic miscarriage with chorionic biopsy (also note Fetal DNA detected at 13 weeks of a Q890X carrier fetus by Gonzalez-Gonzalez MC et al. Prenatal diagnosis 2002; 22:946-948.[PubMed])
Dr Ali Saker is at INSERM, Unité 807, Paris, France, Université Réné Descartes, Paris, France.
Shoseyov D, Brownlee KG, Conway SP, Kerem E. Aspergillus bronchitis in cystic fibrosis. Chest 2006; 130:222-226. [PubMed]

Fig. 42 David Shoseyov
ATS News
A report of six patients with CF with respiratory deterioration that did not respond to appropriate antibiotic treatment. All had had A. fumigatus in their sputum cultures but did not fulfil the accepted criteria of allergic bronchopulmonary aspergillosis (ABPA). Treatment with antifungal agents was followed by improvement in their clinical condition.
The authors suggest that in patients with CF, A. fumigatus should be considered as a pathogen that may directly cause respiratory exacerbations (rather than by only causing an allergic reaction as in ABPA). Antifungal therapy should be considered when deteriorating respiratory function is not responding to antibacterial therapy and A. fumigatus is growing in sputum cultures.Problems with Aspergillus were becoming increasingly common and it is experience in both Leeds and Copenhagen that as the prevalence of chronic Pseudomonas falls, as a result of early eradication therapy, the number of patients growing Aspergillus increases.There is also increasing evidence that, although, at times manifest as typical ABPA, the presence of the fungus in the airways can also cause a subtle deterioration in respiratory function or, as in the present report, even cause an acute bronchial infection amounting to “aspergillus bronchitis” which should be treated with appropriate anti-fungal drugs.
Dr David Shoseyov (Fig. 42) is Senior Physician and Advisor on Pediatric Lung Disease, Hadassah Medical Center, Jerusalem. Department of Pediatrics and CF Center, Hadassah University Hospital, Mount Scopus, Jerusalem, Israel.
Starner TD, Zhang N, Kim G, Apicella MA, McCray PB Jr. Haemophilus influenzae forms biofilms on airway, epithelia: implications in cystic fibrosis. Am J Resp Crit Care Med 2006; 174:213-220. [PubMed]

Fig. 43 Timothy Starner pediatrics.wisc.edu
Non-typeable Haemophilus influenzae (NTHi) commonly infects patients with CF, especially early in childhood and biofilms were common in bronchoalveolar lavage fluid decreasing susceptibility to antibiotics; also respiratory cells exhibited inflammatory and host defence responses – evidence of a dynamic host-pathogen interaction. There are implications both for understanding early CF lung disease pathogenesis and for the treatment of early, asymptomatic colonization of patients with CF with H. influenzae.H. influenzae at times persists in serial respiratory cultures despite antibiotic treatment or soon reappears after such treatment – indicating it has not been eradicated.
It is undoubtedly a pathogen for people with CF who become chronically infected. This warrants vigorous attempts to eradicate the organism, if necessary by intravenous antibiotics if oral treatment fails, an approach analogous to the treatment of early P. aeruginosa. Many CF units would now treat and attempt to eradicate any pathogen grown from the CF airways whether or not there were symptoms, which are a very crude indicator of endobronchial infection.
Timothy D Starner (fig. 43) is a professor and pediatric pulmonologist in Madison at the University of Iowa, Department of Pediatrics, 200 Hawkins Drive, Iowa City, , USA.
Sanders NN, Franckx H, De Boeck K, Haustraete J, De Smedt SC, Demeester J. Role of magnesium in the failure of rhDNase therapy in patients with cystic fibrosis. Thorax 2006; 61:962-968. [PubMed]

Fig 44. Kris De Boeck
rhDNase (Pulmozyme) is a major component of treatment for people with CF but some patients show little or no response and hence fail to benefit from this important drug. In this study the biochemical properties, physical properties, and degradation by rhDNase-1 of sputum obtained from clinical responders and non-responders were compared; also the ability of magnesium to reactivate rhDNase-I in DNA solutions and in sputum was investigated.
The effect of oral magnesium supplements on magnesium levels in the sputum of patients with CF was also examined. Sputum from clinical responders was extensively degraded in vitro on incubation with rhDNase-I, while sputum from clinical non-responders was not degraded: the median decrease in sputum elasticity in the two groups was 32% and 5%, respectively.Sputum from clinical responders contained significantly higher concentrations of magnesium than sputum from non-responders (2.0 mM v 1.3 mM; p = 0.020). Sputum that could not be degraded by rhDNase-I became degradable after preincubation with magnesium. The effect of magnesium on rhDNase-I activity was mediated through actin. Oral intake of magnesium enhanced the magnesium concentration in the sputum of CF patients. So increasing the magnesium concentration in sputum by, for example, oral magnesium supplements may be a promising strategy to overcome the failure of rhDNase-I in some patients with CF.
One further supportive study by Rosenecker J et al. (Airway surface liquid contains endogenous DNase activity which can be activated by exogenous magnesium. Eur J Med Res 2009; 14:304-308. [PubMed]). These authors found BAL samples degraded plasmid DNA only after pre-incubation with magnesium. When analyzing the exhaled breath condensate the samples obtained from the healthy volunteers showed no DNase activity even after pre incubation with magnesium, whereas in one of the two samples obtained from CF patients contained DNase activity after pre-incubation with magnesium.The authors concluded that increasing the magnesium concentration in the airway surface liquid by aerosolisation of magnesium solutions or oral magnesium supplements could improve the removal of highly viscous mucus in chronic lung disease by activating endogenous DNase activity Potentially a very useful paper which could benefit the 30-50% of patients who fail to show a significant response to rhDNase. [PubMed]. .
Professor Kris De Boeck (fig.44) of University Hospital Leuven, Belgium is a leading, very active researcher and clinician in the CF field. Kris De Boeck became President of the ECFS in 2015 when Stuart Elborn retired.
Servetnyk Z, Krjukova J, Gaston B, Zaman K, Hjelte L, Roomans GM, Dragomir A. Activation of chloride transport in CF airway epithelial cell lines and primary CF nasal epithelial cells by S-nitrosoglutathione. Respir Res 2006; 7:124. [PubMed]
The effect of S-nitrosoglutathione (GSNO) on chloride transport was investigated in primary nasal epithelial cells obtained from CF patients homozygous for the delF508 mutation, as well as in two CF cell lines (CFBE and CFSME). Chloride efflux in nasal epithelial cells was increased in 18 out of 21 cells from CF patients, but not in cells from controls.
Previous studies have suggested that treatment with S-nitrosoglutathione promotes maturation of delF508-CFTR, consistent with the results in this study. Here the authors show that GSNO increases chloride efflux, both in the two CF cell lines and in primary nasal epithelial cells from delF508-CF patients. This effect is, at least in part, mediated by CFTR. S-nitrosoglutathione may be a candidate for pharmacological treatment of the defective chloride transport in CF epithelial cells and there has been continuing interest in this possibility. (Also Visca A et al. J Cyst Fibros 2008; 7:433-436. [PubMed]; Corti A et al. 2012; 7:e34772 Epub 2012. [PubMed] free PMC article).
Zhanna Servetnyk is in the Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden.
Serisier DJ, Shute JK, Hockey PM, Higgins B, Conway J, Carroll MP. Inhaled heparin in cystic fibrosis. Eur Respir J 2006; 27:354-358. [PubMed]

Fig 45. Mary Carroll author’s photo
In people with CF inhaled heparin 50,000 IU twice daily had no effect on FEV1, sputum clearance or inflammatory markers. The present authors stated that Heparin thins sputum by decreasing the mucin molecule amino group negative charge, altering its intermolecular hydrogen bonding, and ionically shielding its polyionic moieties. It also has an anti-inflammatory effect within the lung.Ledson et al (Eur Respir J 2001; 17:36-38. [PubMed]) from Liverpool had reported that inhaled heparin had no effect on 6 patients with CF who had B. cepacia infection. As far back as the Seventies Doggett had published a number of papers on reversal of the ciliary inhibition in CF by heparin (Doggett RG, Harrison GM, Patrick TA. 1973.[PubMed]).
– It seems unlikely that this will prove to be a significant advance in treatment. Although the authors suggested that heparin was safe and future evaluation of larger doses over a longer period may be warranted. Further work from Southampton and Portsmouth on cospray-dried unfractionated heparin with L-leucine (to reduce cohesion of the particles) as a dry powder inhaler was published more recently in 2008 (Shur J et al. Drug Dev Ind Pharm 2008; 34:5559-568.[PubMed]) although no further clinical trials in CF have been reported up to 2023.
Dr Mary Carroll (fig.45) is Director of the Southampton Adult CF Centre. There are now 200 patients including some from the Channel Islands. Mary Carroll has a strong research background having helped to develop the technique of nasal ventilation; also more recently work on the use of heparin.
Stephenson A, Jamal S, Dowdell T, Pearce D, Corey M, Tullis E. Prevalence of vertebral fractures in adults with cystic fibrosis and their relationship to bone mineral density. Chest 2006; 130:539-544. [PubMed]

Fig. 46 Anne Stephenson
Cystic Fibrosis Foundation
Seven percent of adult patients with CF had vertebral fractures as determined by morphometry. Subjects in the fracture group had both clinically and statistically higher BMD as measured by DXA. The authors suggest their findings raise the intriguing possibility that BMD may not be useful in identifying CF patients with fractures.
Anne Stephenson (fig. 46) is now a pulmonologist and clinical scientist and Assistant Professor in the Department of Respirology, University of Toronto, ON, Canada.
Slieker MG, van Gestel JP, Heijerman HG, Tramper-Stranders GA, van Berkhout FT, van der Ent CK, Jansen NJ. Outcome of assisted ventilation for acute respiratory failure in cystic fibrosis. Intens Care Med 2006; 32:754-758. [PubMed]

Fig. 47. Martijn Slieker
UMC Utrecht
To assess outcome of assisted ventilation in cystic fibrosis (CF) patients with acute respiratory failure (ARF), to identify risk factors associated with poor outcome and to compare long-term outcome of CF children who were mechanically ventilated for ARF with unventilated CF controls. A retrospective cohort study in two large CF centres in the Netherlands. Thirty-one CF patients required assisted ventilation for ARF between January 1990 and March 2005. All five children (under 2 years of age) and seven adults (27%) survived. CF patients younger than 2 years old, who are ventilated because of ARF, have a good prognosis and their long-term outcome seems identical to unventilated CF controls. ARF in adult CF patients still is associated with high mortality, especially among patients with acute on chronic respiratory failure.
The resort to assisted ventilation has changed over the years now there is the possibility of transplantation; prior to the mid-Eighties before the first successful transplantations ventilation was regarded as inappropriate.
Martijn Slieker (fig. 47) is at the Cystic Fibrosis Centre Utrecht, University Medical Centre Utrecht, and Paediatric Intensive Care Unit, Wilhelmina Children’s Hospital, Utrecht, The Netherlands.
Terribile M, Capuano M, Carnovale V, Ferra P, Petrarulo M, Marangella M. Factors increasing the risk for stone formation in adult patients with cystic fibrosis. Nephrology Dialysis Transplantation 2006; 21:1870-1875. [PubMed]

Fig 48 Maurizio Terribile Calcoli Renali
Detailed metabolic and ultrasound studies of 29 adult patients with CF, 20 heterozygotes (CF-H) and 30 controls (C). 21% of those with CF and 15% of CF-H had kidney stones. Those with CF had elevated uric acid but no other differences compared with heterozygotes and controls. Lower urine volume and acidic pH produced super saturation of CF urine with uric acid in contrast to heterozygotes and controls. The authors considered high risk dietary advice or medication aimed at reducing risk of stones.In another series of people with CF 13% had a history of renal stones – many were recurrent.
People with CF in this present series had a high risk of nephrolithiasis, although we did not recognise this complication through the Eighties although this series was of adults. There have been sporadic reports since 1964 (Gebala A. Pol med Sci Hist Bull 1964; 51:149-154 [PubMed]; Turner et al, 2000 above; Bertenshaw C et al, 2007 for acute renal failure. below). Earlier Bohles & Michalk (Helv Paediatr Acta 1982; 37:267-272.[PubMed]) found patients with CF showed increased urinary concentrations of oxalate, phosphate, xanthine and uric acid, and decreased concentrations of magnesium and citrate, comparable to concentrations found in patients with calcium oxalate stones. However, compared to stone bearing controls their urine calcium concentration was markedly decreased. They suggested that hypocalciuria in CF seems to protect against nephrolithiasis despite the presence of lithogenic factors.
Maurizio Terribile (fig. 48) is in the Department of Nephrology and Renal Stone Centre, Pellgrini Hospital Napoli,

Fig. 49 Joseph Moruousef ResearchGate
There is a more recent review by Moryousef et al, 2021 which records an overall prevalence of 4.6% in the CF population, surgical intervention was required in 37.8% and recurrence occurred in 42.9% [pubmed.ncbi.nlm.nih.gov/33906435/]
Joseph Moryousef (fig 49) is in the Faculty of Medicine, McGill University, Montreal Canada.
Thomson JM, Wesley A, Byrnes CA, Nixon GM. Pulse intravenous methylprednisolone for resistant allergic bronchopulmonary aspergillosis in cystic fibrosis. Pediatr Pulmonol 2006; 41:164-170.pubmed.ncbi.nlm.nih.gov/16317722/

Fig. 50 Alison Wesley
This is the first reported use of pulse intravenous methyl prednisolone in the treatment of ABPA in CF. The authors present the clinical course of four children with CF and severe ABPA, in whom pulse methyl prednisolone was used to manage the disease because of relapses or marked side effects on high-dose oral corticosteroids. Methyl prednisolone pulses achieved disease control in 3 of the 4 children. However, troublesome side effects were experienced, in some cases necessitating discontinuation of therapy.Pulse methyl prednisolone may represent a treatment option for children with CF and ABPA, where ABPA fails to respond adequately to routine therapy.
Dr Janine M Thomson. From the Department of Respiratory Medicine, Starship Children’s Hospital, Auckland, New Zealand. Dr Thomson was subsequently Senior Medical Officer Canterbury District health Board and Clinical Senior Lecturer at the Dept Paediatrics, University of Otago.
Dr Alison Wesley,(Fig 50) the second author, was a pioneer in New Zealand CF care. On a sabbatical to many centres in 1991 Alison visited our CF centre in Leeds. She subsequently invited me (Jim Littlewood) back to New Zealand to visit the CF Centres there.
Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Nat Acad Sc USA 2006; 103:4628-33. [PubMed]

Fig. 51 Rabindra Tirouvanziam ResearchGate
In 18 patients with stable CF, blood neutrophils were deficient in glutathione. In a phase 1 study, this deficiency was improved by the glutathione prodrug N-acetylcysteine, given orally in high doses (0.6 to 1.0 g three times daily, for 4 weeks). This treatment was safe and markedly decreased the sputum elastase activity, the strongest predictor of CF pulmonary function. Consistently, the neutrophil burden in CF airways was decreased upon treatment, as was the number of airway neutrophils actively releasing elastase-rich granules, as measured by flow cytometry. Thus, high-dose oral N-acetylcysteine has the potential to counter the intertwined redox and inflammatory imbalances in CF.
Dr Rabindra Tirouvanziam (fig.51) is Assistant Professor of Pediatrics in the Department of Genetics, Stanford University School of Medicine, Stanford, CA 94305, USA.
— The story of acetylcysteine in CF is quite extraordinary. It has been used as a mucolytic in chronic respiratory conditions since the Sixties (Sheffner AL. Ann N Y Acad Sci 1963; 106:298-310 [PubMed];Webb WR. Clinical evaluation of a new mucolytic agent acetyl-cysteine. J Thorac Cardiovasc Surg 1962; 44:330-343 above. [PubMed]) . Although not recommended or widely used in CF as a sputum mucolytic in the UK, the drug has been used in CF for recurrent distal intestinal obstruction.
The relation to glutathione in particular has featured in recent references as N-acetylcysteine is an effective precursor of cysteine for tissue glutathione synthesis. Apparently CFTR is responsible for glutathione transport across cell membranes and there may be intracellular accumulation of glutathione in cystic fibrosis (Childers M. Med Hypotheses 2007; 68:101-102 below.[PubMed]).
However, a recent Cochrane review, by very experienced reviewers, concluded – “we found no evidence to recommend the use of either nebulised or oral thiol derivatives in people with cystic fibrosis. There are very few good quality trials investigating the effect of these medications in cystic fibrosis, and further research is required to investigate the potential role of these medications in improving the outcomes of people with cystic fibrosis”. Nash EF, Stephenson A, Ratjen F, Tullis E. Nebulizer and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No.: CD007168. DOI: 10.1002/14651858.CD007168.pub2.
Nevertheless, in 2012 another CF Foundation clinical trial in people with CF in progress. Also it is reported that N-acetylcysteine enhances the CF sputum penetration and airway gene transfer by highly compacted nanoparticles (Suk JS et al. Mol Ther 2011; 19:1981-1989. [PubMed])
van Ewijk BE, Wolfs TF, Fleer A, Kimpen JL, van der Ent CK. High Pseudomonas aeruginosa acquisition rate in CF. Thorax 2006l; 61:641-642. [PubMed] Full text available

Fig 52. Cornelius K Van Der Ent
Authors performed systematic oropharyngeal cultures during periods of ARI between November and May in 20 young children with CF of mean (SD) age 3.6 (2.0) years (range 0.1–7.4) and 19 unrelated age matched healthy controls of mean (SD) age 3.6 (1.7) years. During the study period six children with CF (30%) had at least one P aeruginosa positive culture compared with seven (37%) healthy controls. Cultures following a positive culture in healthy children were always negative for P aeruginosa, while in four of six (67%) CF children short term follow up cultures remained positive for P aeruginosa and anti‐pseudomonal treatment was started.
— These are new findings and suggest that non-CF children with viral colds may be a source of P .aeruginosa. They also explain previous findings from Copenhagen that P. aerginosa is more commonly isolated in the viral season.
Professor Kors (Cornelis K) van der Ent (fig. 52) is a paediatrician and Professor in Paediatric Pulmonology. After medical training in Rotterdam and Utrecht he became a research fellow at the department of Paediatric Respiratory Diseases where he graduated in 1997 on a thesis on clinical and physiological aspects of tidal breathing analysis as a measure for airway obstruction in young infants. At the same year he founded the Utrecht Cystic Fibrosis Center, being the largest in the country. In 2000 he became head of the department of Paediatric Respiratory Diseases and Allergology. He has published widely on both respiratory conditions of children and cystic fibrosis.
Vidal V, Therasse E, Berthiaume Y, Bommart S, Giroux MF, Oliva VL, Abrahamowicz M, du Berger R, Jeanneret A, Soulez G. Bronchial artery embolization in adults with cystic fibrosis: impact on the clinical course and survival. J Vasc Intervent Radiol 2006; 17:953-958. [PubMed]

Fig 53. Gilles Soulez
Of 297 patients with cystic fibrosis hospitalized from 1990 to 2004, 30 patients (mean age, 26.7+/-9.2 years) presented with major or persistent hemoptysis that required 42 BAE sessions. These patients were compared with a control group of 27 patients without hemoptysis requiring embolization who were matched for age, sex, and forced expiratory volume in 1 second (FEV1). Hemoptysis stopped within 24 hours after BAE in 96.6% of patients (n=29), and there were no major complications. The change in the slope of FEV1 after the BAE or matching date was significantly worse in the embolization group (P=.0007). At last follow-up, nine and one patients, respectively, had undergone lung transplantation in the BAE and control groups (P=.002). The 5-year survival rates without lung transplantation were 31% and 84%, respectively, in the BAE and control groups (hazard ratio, 5.95; P=.002). Sixty-two percent of patients were free of hemoptysis 5 years after BAE. The number of collateral arteries was the only factor associated with the risk of death or recurrent hemoptysis (P=.001).Despite the effectiveness of embolization in controlling recurrent or major hemoptysis, adults with cystic fibrosis who have undergone BAE for hemoptysis are at much higher risk of respiratory function aggravation, death, and the need for lung transplantation than those who have not undergone BAE for hemoptysis. They are more likely to die or to undergo lung transplantation than to present with recurrent major hemoptysis.
This is extensive and successful experience of treating haemoptysis in people with CF. As to be expected the patients with haemoptysis had more severe chest involvement and generally a worse prognosis but the procedure was successful and free of major complications with regard to controlling the bleeding.
Professor Gilles Soulez (fig.53) is Professor in the Department of Radiology at the Centre Hospitalier de L’Universite de Montreal and has published widely on various aspects of interventional radiology relating to the cardiovascular system.
Williams V, Riley A, Rayner R, Richardson K. Inhaled nitrous oxide during painful procedures: a satisfaction survey. Paediatric Nursing 2006; 18:31-33.[PubMed]
Fix 54. Rosie Raynor author’s photo
Inhaled nitrous oxide was safe and effective in reducing trauma and the effects of needle phobia and being offered to children with CF for procedural pain in the district general hospital at Wolverhampton.
Dr Rosie Rayner (fig 54), the paediatrician involved in this study, was previously the CF Trust Research Fellow in Nottingham. The use of nitrous oxide had been previously reported at CF Meetings by this team from Wolverhampton (Williams V et al, J Cyst Fibros 2004; 3: S100 Poster 376; also Maddison JC et al, Poster 377; original report by Mills HL & Redmond AOB, 2001 above).
Wood DM, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database of Systematic Reviews. (1):CD004197, 2006. [PubMed]

Fig. 55 Damion Wood
eMedEvents
This search identified 15 trials on the subject but the reviewers considered that only 3 trials, involving 69 participants, were eligible for inclusion. The reviewers considered that there was evidence from two randomised controlled trials, of “questionable methodological quality”, that treatment of early P. aeruginosa infection with inhaled tobramycin resulted in microbiological eradication of the organism from respiratory secretions more often than placebo and that this effect may persist for up to 12 months, however incomplete data from one of the trials precluded an accurate analysis.
One randomised controlled trial of oral ciprofloxacin and nebulised colistin versus usual treatment was identified (Valerius et al, 1991 above) but the reviewers considered this trial was of poor methodological quality. The results suggested treatment of early infection results in microbiological eradication of P. aeruginosa more often than usual treatment, after two years.
The reviewers considered that there was insufficient evidence to determine whether antibiotic strategies for the eradication of early P. aeruginosa decrease mortality or morbidity, improve quality of life, or are associated with adverse effects compared to placebo or standard treatment. From the three trials included in this review, there was some evidence that antibiotic treatment of early P. aeruginosa results in short-term eradication but it remains uncertain whether there is clinical benefit to people with cystic fibrosis.
Dr Damion Wood (fig.55) is a paediatrician in Nottingham.
– The conclusions of this Cochrane review were quite out of keeping with the clinical experience of most experienced CF clinicians and the study was soundly criticised by Prof. Niels Hoiby who regarded further trials as unwarranted.
The opinion of an experienced and respected clinician such as the late Christian Koch was representative of current opinion (quoted in entry on Valerius et al, 1991 above). By the time of this review (2006) there was already a wealth of evidence that avoidance of chronic P. aeruginosa infection improved clinical condition and increased survival – evidence that the reviewers did not consider (Kerem et al, 1990; Henry et al, 1992; Hudson et al, 1993; Pamucku et al, 1995 above; Frederiksen et al, 1996; CF Foundation Patient Registry 1996; Frederiksen et al, 1997 above; Kosorok et al, 2001). Presumably this evidence was ignored as it did not directly concern eradication of P. aeruginosa.
It is important to stress that eradication of early P. aeruginosa infection, to prevent or delay chronic infection, is one of the most important aspects of therapy and acquisition of chronic infection should now be regarded as avoidable and represent a failure of therapy (Drittanti et al, 1996 above).
A further Cochrane review on this subject assessed as up to date in August 2009 identified 25 trials but only considered 4 eligible for inclusion !! The authors concluded that “treatment with nebulised antibiotics alone or in combination with oral antibiotics was better than no treatment for early infection with P. aeruginosa. Eradication may be sustained in the short term. Overall there is insufficient evidence from this review to state which antibiotic strategy should be used for the eradication of early P. aeruginosa infection in CF”. (SC Langton-Hewer, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No.: CD004197. DOI: 10.1002/14651858.CD004197.pub3

Fig. 56 Simon Langton-Hewer
The most recent Cochrane Review on this subject in 2017 by Simon Langton Hewer and Alan Smyth concludes –
“We found that nebulised antibiotics, alone or in combination with oral antibiotics, were better than no treatment for early infection with Pseudomonas aeruginosa. Eradication may be sustained for up to two years. There is insufficient evidence to determine whether antibiotic strategies for the eradication of early Pseudomonas aeruginosa decrease mortality or morbidity, improve quality of life, or are associated with adverse effects compared to placebo or standard treatment (?). Four trials comparing two active treatments have failed to show differences in rates of eradication of Pseudomonas aeruginosa. There have been no published randomised controlled trials that investigate the efficacy of intravenous antibiotics to eradicate Pseudomonas aeruginosa in cystic fibrosis (vide infra). Overall, there is still insufficient evidence from this review to state which antibiotic strategy should be used for the eradication of early Pseudomonas aeruginosa infection in cystic fibrosis”.
Finally a subsequent and valuable major randomised controlled trial, coordinated by Simon Langton Hewer, comparing intravenous and inhaled antibiotics for eradication of P. aeruginosa was completed in 2021 and showed no difference between the two methods of eradication. https://pubmed.ncbi.nlm.nih.gov/34806975/
Dr Simon Langton-Hewer (Fig. 56) is a consultant paediatrician in Bristol, UK.
Yahav J, Samra Z, Blau H, Dinari G, Chodick G, Shmuely H. Helicobacter pylori and Clostridium difficile in cystic fibrosis patients. Digest Dis Sci 2006; 51:2274-9. [PubMed]
Stool specimens from 30 consecutive patients with CF, aged 1-44, and from 30 healthy similarly aged subjects were tested for the H. pylori antigen by specific monoclonal antibodies and for CD toxins by Tox A/B assay and Tox A assay. CF patients were assessed clinically and tested for specific H. pylori serum antibodies and for mutations. In CF patients, the prevalence of H. pylori antigen was 16.6% (5/30), compared to 30% (9/30) in controls. Of the 26 CF patients with PI, only 2 (7.6%) were infected by H. pylori, compared with 3 of the 4 (75%) patients with PS (P=0.001). H. pylori infection was diagnosed in 3 of 5 (60%) CF patients carrying mild mutations, compared to 1 of 25 (4%) CF patients carrying severe mutations (P=0.01). Fourteen of 30 (46.6%) stool specimens from CF patients tested positive in the ToxA/B assay, and 3 of 14 tested positive for ToxA. No significant differences in antibiotic use, severity of lung disease, PI, chronic abdominal pain, or genotype were found between the two groups. None of the controls was positive for CD toxins. Prevalence of H. pylori infection in CF patients was lower than in similarly aged non-CF controls. CF patients with PI or a history of distal intestinal obstruction syndrome and those carrying mutations associated with a severe phenotype were protected against H. pylori infection. Almost half of the CF patients were asymptomatic carriers of CD producing mostly toxin B.
The authors suggested more studies were needed to confirm their results in a larger group of CF patients.
However, this paper does confirms the previously reported low incidence of H. pylori in people with CF.
Dr J Yahav is at the Helicobacter Research Institute, Rabin Medical Center, Beilinson Campus, Petah Tikva, Israel.
Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest 2006; 130:995-1002. [PubMed]

Fig. 57 Thoamsz Ziedalski
LinkedIn
Fifty adult patients at Stanford University Medical Center with a diagnosis of bronchiectasis and/or pulmonary NTM infection were prospectively characterized by sweat chloride measurement, comprehensive mutational analysis of CFTR, and sputum culture results.
A new diagnosis of cystic fibrosis (CF) was established in 10 patients (20%). Sweat chloride concentration was elevated to > 60 mEq/dL in seven patients (14%), and from 40 to 60 mEq/dL in eight patients (16%). The frequency of CFTR mutations was elevated above that expected in the general population: heterozygous DeltaF508 (12% vs 3%), R75Q (14% vs 1%), and intron 8 5T (17% vs 5 to 10%).
It does appear that there is an increase in CFTR mutations in patients with chronic respiratory conditions when comprehensive mutational analysis is used.
Dr Tomasz M Ziedalki (fig.57) is a pulmonologist in Everett, Washington.
THE FOLLOWING PAPERS ON VX-770 AND VX-809 ARE INCLUDED TOGETHER IN THIS SECTION. THEY ARE ALSO INCLUDED IN THE DRUGS SECTION OF “TOPICS”
2006 Van Goor F. Straley KS. Cao D. Gonzalez J. Hadida S. Hazlewood A. Joubran J. Knapp T. Makings LR. Miller M. Neuberger T. Olson E. Panchenko V. Rader J. Singh A. Stack JH. Tung R. Grootenhuis PD. Negulescu P.Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol_Lung C 2006; 290:L1117-30.[PubMed]

Fig 58. F Van Goor
Here, van Goor and colleagues describe two classes of novel, potent small molecules identified from screening compound libraries that restore the function of DeltaF508-CFTR in both recombinant cells and cultures of human bronchial epithelia isolated from CF patients.
The first class partially corrects the trafficking defect by facilitating exit from the endoplasmic reticulum and restores DeltaF508-CFTR-mediated Cl(-) transport to more than 10% of that observed in non-CF human bronchial epithelial cultures, a level expected to result in a clinical benefit in CF patients.
The second class of compounds potentiates cAMP-mediated gating of DeltaF508-CFTR and achieves single-channel activity similar to wild-type CFTR. The CFTR-activating effects of the two mechanisms are additive and support the rationale of a drug discovery strategy based on rescue of the basic genetic defect responsible for CF.
Dr Frederick van Goor (fig.58)) is Head of Biology CF Research Program, Vertex Pharmaceuticals. There are a number of accounts on the internet of the development of the Kalydeco by Vertex with considerable scientific, clinical and financial support from the CF Foundation.
The article by Matthew Herper in Forbes Magazine describes van Goor’s early involvement with the research.
2008 Accurso FJ, Rowe SM, Durie PR, Konstan MW, Dunitz J, Hornick DB, Sagel SD, Boyle MP, Uluer AZ, Upadhyay D, Ramsey BW, Freedman SD, Dong Q, Ahmed AM, Stone AJ, Olson ER, Ordenez CL, Clancy JP, Campbell PW, Ashlock MA. Interim results of Phase IIa study of VX-770 to evaluate safety, pharmokinetics and biomarkers of CFTR activity in cystic fibrosis subjects with G551D. Pediatr Pulmonol 2008; Suppl 31: 267 page 295. (Poster)
Results reported at the 2008 North American CF Conference of an interim analysis in 20 patients with the G551D mutation who received 14 days of oral VX-770, a potentiator of CFTR, 25mg, 75mg or 150mg 12 hourly or placebo. Those on highest dose of VX-770 (150mg twice daily) had an impressive response. They had increases in FEV1 of 10.1%, their sweat chloride fell from a mean of 95.5 to 53.2 mmol/l, nasal potential difference showed significant changes towards normal.(-5.4mv in treated CF with -1.7mv in controls).
This was an early but very encouraging result arising from the CF Foundation’s search for chemical activators of mutant CFTR. The change in sweat electrolytes is particularly impressive and this is the first drug to affect the level of sweat electrolytes in people with CF.

Fig 59. Frank Accurso
A Phase II trial with 16 patients for 28 days started in 2008 and impressive progress was reported in 2009 (Boyle et al. Pediatr Pulmonol 2009; Suppl 32:287. Poster 217).
Dr Frank Accurso (fig. 59) is Professor of Pediatrics and Head of Pulmonary Medicine in the University of Colorado. Following the selection of the University of Colorado as one of the Cystic Fibrosis Foundation’s Therapeutics Development Centers, Dr Accurso has been very active in the development and study of various new therapies and techniques for outcomes research2010
2010 Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Doladson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q,Zha J, Stone AJ, Olson ER, Ordonez CL, Campbell PW, Ashlock MA, Ramsay BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010; 363:1991-2003. [PubMed].
The authors randomly assigned 39 adults with cystic fibrosis and at least one G551D-CFTR allele to receive oral VX-770 every 12 hours at a dose of 25, 75, or 150 mg or placebo for 14 days (in part 1 of the study) or VX-770 every 12 hours at a dose of 150 or 250 mg or placebo for 28 days (in part 2 of the study).
At day 28, in the group of subjects who received 150 mg of VX-770, the median change in the nasal potential difference (in response to the administration of a chloride-free isoproterenol solution) from baseline was -3.5 mV (range, -8.3 to 0.5; P=0.02 for the within-subject comparison, P=0.13 vs. placebo), and the median change in the level of sweat chloride was -59.5 mmol per liter (range, -66.0 to -19.0; P=0.008 within-subject, P=0.02 vs. placebo). The median change from baseline in the percent of predicted forced expiratory volume in 1 second was 8.7% (range, 2.3 to 31.3; P=0.008 for the within-subject comparison, P=0.56 vs. placebo). None of the subjects withdrew from the study. Six severe adverse events occurred in two subjects (diffuse macular rash in one subject and five incidents of elevated blood and urine glucose levels in one subject with diabetes). All severe adverse events resolved without the discontinuation of VX-770.
The authors concluded that their study to evaluate the safety and adverse-event profile of VX-770 showed that VX-770 was associated with within-subject improvements in CFTR and lung function.
– These impressive findings provided support for further studies of pharmacologic potentiation of CFTR as a means to treat cystic fibrosis and heralded a new era in CF treatment.
2011 Ramsey BW. Davies J. McElvaney NG. Tullis E. Bell SC. Drevinek P. Griese M. McKone EF. Wainwright CE. Konstan MW. Moss R. Ratjen F. Sermet-Gaudelus I. Rowe SM. Dong Q. Rodriguez S. Yen K. Ordonez C. Elborn JS. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Eng J Med 2011; 365:1663-1672.[PubMed]

Fig. 60 Bonnie Ramsey
A randomized, double-blind, placebo-controlled trial to evaluate ivacaftor (VX-770), a CFTR potentiator, in subjects 12 years of age or older with cystic fibrosis and at least one G551D-CFTR mutation. Subjects were randomly assigned to receive 150 mg of ivacaftor every 12 hours (84 subjects, of whom 83 received at least one dose) or placebo (83, of whom 78 received at least one dose) for 48 weeks. The primary end point was the estimated mean change from baseline through week 24 in the percent of predicted forced expiratory volume in 1 second (FEV(1)).
RESULTS: The change from baseline through week 24 in the percent of predicted FEV(1) was greater by 10.6 percentage points in the ivacaftor group than in the placebo group (P<0.001). Effects on pulmonary function were noted by 2 weeks, and a significant treatment effect was maintained through week 48. Subjects receiving ivacaftor were 55% less likely to have a pulmonary exacerbation than were patients receiving placebo, through week 48 (P<0.001). In addition, through week 48, subjects in the ivacaftor group scored 8.6 points higher than did subjects in the placebo group on the respiratory-symptoms domain of the Cystic Fibrosis Questionnaire-revised instrument (a 100-point scale, with higher numbers indicating a lower effect of symptoms on the patient’s quality of life) (P<0.001). By 48 weeks, patients treated with ivacaftor had gained, on average, 2.7 kg more weight than had patients receiving placebo (P<0.001). The change from baseline through week 48 in the concentration of sweat chloride, a measure of CFTR activity, with ivacaftor as compared with placebo was -48.1 mmol per liter (P<0.001). The incidence of adverse events was similar with ivacaftor and placebo, with a lower proportion of serious adverse events with ivacaftor than with placebo (24% vs. 42%).
The authors concluded that ivacaftor was associated with improvements in lung function at 2 weeks that were sustained through 48 weeks. Substantial improvements were also observed in the risk of pulmonary exacerbations, patient-reported respiratory symptoms, weight, and concentration of sweat chloride. A quite remarkable result.
The FDA statement (Jan 2012) reads – “The U.S. Food and Drug Administration today approved Kalydeco (ivacaftor) for the treatment of a rare form of cystic fibrosis (CF) in patients ages 6 years and older who have the specific G551D mutation in the Cystic Fibrosis Transmembrane Regulator (CFTR) gene. … Two 48-week, placebo-controlled clinical studies involving 213 patients, one in patients ages 12 years and older and another in patients ages 6 years to 11 years, were used to evaluate the safety and efficacy of Kalydeco in CF patients with the G551D mutation. In both studies, treatment with Kalydeco resulted in significant and sustained improvement in lung function”.
2011 VX-809 – a new CFTR corrector
Van Goor F. Hadida S. Grootenhuis PD. Burton B. Stack JH. Straley KS. Decker CJ. Miller M. McCartney J. Olson ER. Wine JJ. Frizzell RA. Ashlock M. Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by investigational drug VX-809. Proc Natl Acad Sci USA 2011; 108:18843-8.[PubMed]
Most patients with CF carry the F508del CFTR mutation, which causes defective CFTR protein folding and processing in the endoplasmic reticulum, resulting in minimal amounts of CFTR at the cell surface. One strategy to treat these patients is to correct the processing of F508del-CFTR with small molecules. Here the authors describe the in vitro pharmacology of VX-809, a CFTR corrector that was advanced into clinical development for the treatment of CF.
In cultured human bronchial epithelial cells isolated from patients with CF homozygous for F508del, VX-809 improved F508del-CFTR processing in the endoplasmic reticulum and enhanced chloride secretion to approximately 14% of non-CF human bronchial epithelial cells (EC(50), 81 +/- 19 nM), a level associated with mild CF in patients with less disruptive CFTR mutations.
F508del-CFTR corrected by VX-809 exhibited biochemical and functional characteristics similar to normal CFTR, including biochemical susceptibility to proteolysis, residence time in the plasma membrane, and single-channel open probability. VX-809 was more efficacious and selective for CFTR than previously reported CFTR correctors. VX-809 represents a class of CFTR corrector that specifically addresses the underlying processing defect in F508del-CFTR.A report showing some degree of in vitro activity.
2012 Clancy JP. Rowe SM. Accurso FJ. Aitken ML. Amin RS. Ashlock MA. Ballmann M. Boyle MP. Bronsveld I. Campbell PW. De Boeck K. Donaldson SH. Dorkin HL. Dunitz JM. Durie PR. Jain M. Leonard A. McCoy KS. Moss RB. Pilewski JM. Rosenbluth DB. Rubenstein RC. Schechter MS. Botfield M. Ordonez CL. Spencer-Green GT. Vernillet L. Wisseh S. Yen K. Konstan MW. Results of a Phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508Del-CFTR mutation. Thorax 2012; 67:12-18. [PubMed]
VX-809, a cystic fibrosis transmembrane conductance regulator (CFTR) modulator, has been shown to increase the cell surface density of functional F508del-CFTR in vitro (Van Goor et al, 2011.above)
A randomised, double-blind, placebo-controlled study evaluated the safety, tolerability and pharmacodynamics of VX-809 in adult patients with cystic fibrosis (n=89) who were homozygous for the F508del-CFTR mutation. Subjects were randomised to one of four VX-809 28 day dose groups (25, 50, 100 and 200 mg) or matching placebo.
The type and incidence of adverse events were similar among VX-809- and placebo-treated subjects. Respiratory events were the most commonly reported and led to discontinuation by one subject in each active treatment arm. Pharmacokinetic data supported a once-daily oral dosing regimen.
Pharmacodynamic data suggested that VX-809 improved CFTR function in at least one organ (sweat gland). VX-809 reduced elevated sweat chloride values in a dose-dependent manner (p=0.0013) that was statistically significant in the 100 and 200 mg dose groups. There was no statistically significant improvement in CFTR function in the nasal epithelium as measured by nasal potential difference, nor were there statistically significant changes in lung function or patient-reported outcomes. No maturation of immature F508del-CFTR was detected in the subgroup that provided rectal biopsy specimens.
The authors concluded that VX-809 had a similar adverse event profile to placebo for 28 days in F508del-CFTR homozygous patients, and demonstrated biological activity with positive impact on CFTR function in the sweat gland. Additional data were needed to determine how improvements detected in CFTR function secondary to VX-809 in the sweat gland relate to those measurable in the respiratory tract and to long-term measures of clinical benefit.

