Introduction
Although in the early stages of cystic fibrosis (CF) there may be few or no clinical abnormalities, bronchoscopy studies have documented respiratory infection and inflammation in well infants (Armstrong et al, 1995; Khan et al, 1995; Armstrong et al, 1996; Rosenfeld et al, 2001).
Viral and bacterial infections with Staphylococcus aureus and Haemophilus influenzae predominate in younger patients. Chronic infection with P. aeruginosa used to be common in most patients by their late teens. This is no longer true. Less than 20% of our paediatric (Lee et al, 2003) and about 55% of our adult population have chronic P. aeruginosa infection. The impact of this epidemiological change can be seen in the outpatient clinics where one to two sessions each month are reserved for patients free of P. aeruginosa infection in the adult clinic, and only two clinics a month are designated for children with chronic P. aeruginosa infection. Chronic infection can be prevented, or at least be substantially delayed by intensive treatment of the first P. aeruginosa isolate, using an ‘eradication regimen’ of antibiotics, often combinations of nebulised, oral and/or intravenous antibiotics (Vazquez et al, 1993; Frederiksen et al, 1997; Ratjen et al, 2001; Taccetti et al, 2005; Lebecque et al, 2006). The most effective regimen is still uncertain (Conway et al, 2003). It is critically important to offer this treatment to all patients because chronic P. aeruginosa infection results in worse clinical status, lower respiratory function, lower weight, height and BMI, more hospital admissions and increased treatment costs (Konstan et al, 1999; Emerson et al, 2002; Taccetti et al, 2005). In the majority of patients chronic P. aeruginosa infection is with the mucoid form of the bacteria. This defends itself against ingestion and destruction by the blood’s white cells by producing large amounts of a protective film/mucus called alginate (Lyczak et al, 2002). Alginate also protects P. aeruginosa against the activity of antibiotics (Aaron et al, 2002). The huge numbers of peripheral white blood cells which congregate in the lung in an attempt to kill these bacteria release powerful enzymes, directed at the bacteria but also damaging to the lung itself. These fuel a continuing inflammatory process in the lungs, the end result of which is destruction of lung tissue (Venaille et al, 1998). Following repeated respiratory tract infections it is the body’s response to P. aeruginosa infection which is largely responsible for causing lung damage (Heeckeren et al, 1997; Konstan & Berger, 1997).
Eradication regimens for early or recurrent P. aeruginosa infection
Leeds criteria for P. aeruginosa infection:
Chronic infection: P. aeruginosa isolated in more than 50% of months when sputum, cough or throat swab taken in previous 12 month period
Intermittent infection: P. aeruginosa isolated in 50% or less months when sputum, cough swab or throat swab sample taken in previous 12 month period
Free of infection: P. aeruginosa isolated previously but not for 12 months or more
Never infected: P. aeruginosa never isolated
Background
Chronic P. aeruginosa infection is associated with the mucoid phenotype of P. aeruginosa (Lyczak et al, 2002), a worse prognosis and a more rapid decline in lung function. Strategies aimed at preventing or delaying progression from initial acquisition of P. aeruginosa to chronic infection are an essential part of CF management and have proved highly effective. Recent data suggest that the window of opportunity for such strategies may be quite large (Li et al, 2005). Whilst acquisition of P. aeruginosa may occur quite early in life, the transition from the non-mucoid to the mucoid phenotype may take several years.
Evidence from several studies has shown that early administration of antibiotics, once colonisation with P. aeruginosa has been identified, significantly reduces the risk of chronic infection (Littlewood et al, 1985; Valerius et al, 1991; Frederiksen et al, 1997; Ratjen et al, 2001; Gibson et al, 2003; Taccetti et al, 2005; Lebecque et al, 2006). A common feature of these trials is the use of aerosolised antibiotics. Conversely trials using intravenous antibiotics as early eradication therapy have been disappointing (Steinkamp et al, 1989).
In the study conducted by Valerius et al, 26 patients received oral ciprofloxacin plus aerosolised colistin twice daily for three weeks or no treatment, in response to an initial isolation of P. aeruginosa. After 27 months of the trial significantly fewer patients who had received treatment were positive for P. aeruginosa (14% vs. 58%, p<0.05), (Valerius et al, 1991). This protocol was further refined by the Copenhagen group to increase the duration of treatment with oral ciprofloxacin and aerosolised colistin to three months. Further follow-up after three and a half years revealed that only 16% of treated patients developed chronic P. aeruginosa infection in comparison to 72% of untreated historical controls (p<0.005), (Frederiksen et al, 1997). Ratjen et al gave 15 patients aerosolised tobramycin 80mg twice daily for 12 months in response to initial colonisation with P. aeruginosa (Ratjen et al, 2001). After one year of follow-up, 14 of 15 patients remained negative for P. aeruginosa. Gibson et al randomised 21 children under the age of six years who became positive for P. aeruginosa to receive 300mg Tobramycin Solution for Inhalation (TOBI®) or placebo twice daily for 28 days (Gibson et al, 2003). At the end of treatment all eight children who received TOBI® vs. one of 13 who received placebo (p<0.0001) were negative for P. aeruginosa.
Early eradication therapy is believed to be a major reason for the increased survival of patients with CF, mainly as a result of reduction in the prevalence of chronic P. aeruginosa infection (Johansen et al, 2004; Lee et al, 2004).
The Leeds eradication regimen
There have been no studies comparing colistin with TOBI® for early eradication of new isolates of P. aeruginosa. While there is evidence for the efficacy of TOBI®, the cheaper alternative colistin has been extensively used and has proved highly effective when combined with oral ciprofloxacin in preventing, or at least substantially delaying, the first P. aeruginosa isolate (Valerius et al, 1991; Frederiksen et al, 1997). In our centre we use three months of nebulised colistin (2MU bd) in combination with oral ciprofloxacin (35-50mg/kg/day in two divided doses) on first isolation of P. aeruginosa (Figure 1). For the first month we also prescribe azithromycin to hopefully increase the success rate by decreasing both P. aeruginosa adherence to the respiratory epithelium and biofilm growth. We prescribe 500mg or 250mg azithromycin daily on Monday, Wednesday and Friday depending on whether the patient’s weight is greater or less than 40kg. For children less than eight years old we prescribe 10mg/kg once daily on Monday, Wednesday and Friday. We are presently auditing the effectiveness of this additional treatment. TOBI® is substituted for patients intolerant of nebulised colistin.
If the above first line treatment fails or there is an early pseudomonas recurrence we prescribe two weeks of intravenous anti-pseudomonal antibiotics followed by a further three months of nebulised TOBI® and oral ciprofloxacin, and a further nine months of on/off TOBI®.
Patients whose new pseudomonas isolate is associated with a respiratory exacerbation, however mild, are treated with two weeks of intravenous anti-pseudomonal antibiotics before starting one month of oral azithromycin and three months of nebulised colistin and oral ciprofloxacin.
We treat an acute rise in P. aeruginosa antibodies in a patient from whom pseudomonas has not been isolated for more than a year with the above protocol even in the absence of a new pseudomonas growth.
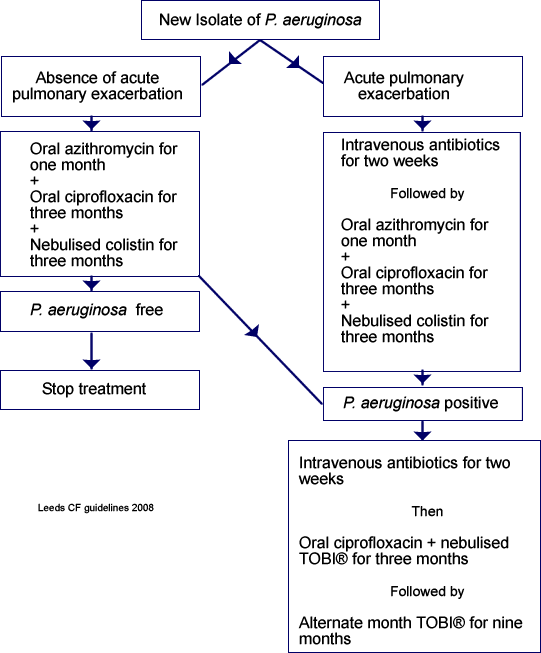
Figure 1. The protocol for P. aeruginosa eradication followed in the Leeds CF Unit
Safety concernsEradication therapy is usually well-tolerated. Absorption of aerosolised tobramycin does not reach sufficient levels in the majority of patients to affect renal function. A retrospective review of children with CF receiving inhaled gentamicin showed significantly raised urinary N-acetyl-b-D-glucosaminidase (NAG) activity indicating renal tubular damage compared to control children who had never received inhaled gentamicin or who had discontinued the drug at least three months previously (Ring et al, 1998). The long term clinical implication of these findings remained uncertain as urinary NAG activity returned to normal at the end of treatment.
There has been no evidence to suggest significant increases in antimicrobial resistance during eradication therapy, even after multiple repeat courses (Ho et al, 2004). Aerosolised antibiotics and oral ciprofloxacin have been associated with an increased risk of colonisation with S. maltophilia (Denton et al, 1996), Aspergillus species (Bargon et al, 1999) and MRSA (Nadesalingam et al, 2005).
Monitoring
We recommend that all patients who are free of P. aeruginosa or intermittent growers should be regularly monitored using pseudomonas antibody titres (three monthly) and regular sputum or cough swab specimens (one to two monthly). Eradication therapy will only be effective if appropriate microbiological surveillance is undertaken.
Key points
• Chronic P. aeruginosa infection can be significantly delayed or prevented
• Chronic P. aeruginosa infection is associated with a worse prognosis and a more rapid decline in lung function
• Early eradication therapy is a major reason for increased patient survival, mainly as a result of a reduction in the prevalence of chronic P. aeruginosa infection
Recommendations
• First line therapy should be based on a regimen including three months of nebulised colistin in combination with oral ciprofloxacin
• If first line treatment fails we recommend two weeks of intravenous anti-pseudomonal antibiotics followed by three months of nebulised TOBI® and oral ciprofloxacin, and nine months of on/off TOBI®
• Patients presenting with a new growth of P. aeruginosa and a respiratory exacerbation should receive two weeks of intravenous anti-pseudomonal antibiotics before the three month regimen
• TOBI® should be substituted for colistin for patients intolerant of nebulised colistin
References
Aaron SD, Ferris W, Ramotar K, et al. Single and combination antibiotic susceptibilities of planktonic, adherent and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J Clin Microbiol 2002; 40: 4172-4179. [PubMed]
Armstrong DS, Grimwood K, Carzino R et al. Lower respiratory tract infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995; 310: 1571-1572. [PubMed]
Armstrong DS, Grimwood K, Carlin JB et al. Bronchoalveolar lavage and oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol 1996; 21: 267-275. [PubMed]
Bargon J, Dauletbaev N, Kohler B, et al. Prophylactic antibiotic therapy is associated with an increased prevalence of Aspergillus colonization in adult cystic fibrosis patients. Respir Med 1999; 93: 835-838. [PubMed]
Conway SP, Brownlee KG, Denton M, et al. Antibiotic treatment of multidrug-resistant organisms in cystic fibrosis. Am J Respir Med 2003; 2: 321-332.[PubMed]
Denton M, Todd NJ, Littlewood JM. Role of anti-pseudomonal antibiotics in the emergence of Stenotrophomonas maltophilia in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis 1996; 15: 402-405. [PubMed]
Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34: 91-100. [PubMed]
Frederiksen B, Koch C, Hoiby N. Antibiotic treatment at time of initial colonisation with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration in pulmonary function in patients with cystic fibrosis. Pediatr Pulmonol 1997; 23: 330-335. [PubMed]
Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003; 167: 841-849. [PubMed]
Heeckeren A, Walenga R, Konstan MW, et al. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997; 100: 2810-2815. [PubMed]
Ho SA, Lee TWR, Denton M, et al. Successful antibiotic eradication of Pseudomonas aeruginosa infection does not promote drug resistance in subsequent re-growths of this bacterium in children. J Cyst Fibros 2004; 3(Suppl 1): S34.
Johansen HK, Hoiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis. Thorax 1992; 47: 109-111. [PubMed]
Johansen HK, Norregaard L, Gotzsche PC, et al. Antibody response to Pseudomonas aeruginosa in cystic fibrosis patients: a marker of therapeutic success? Pediatr Pumonol 2004; 37: 427-432. [PubMed]
Khan TZ, Wagener JS, Bost T, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995; 151: 1075-1082. [PubMed]
Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and aetiology. Pediatr Pulmonol 1997; 24: 137-142. [PubMed]
Konstan MW, Butler SM, Schidlow DV, et al. Patterns of medical practice in cystic fibrosis. Pediatr Pulmonol 1999; 28: 242-247. [PubMed]
Lebecque P, Leal T, Zylberberg K, et al. Towards zero prevalence of chronic Pseudomonas aeruginosa infection in children with cystic fibrosis. J Cyst Fibros 2006; 5: 237-244. [PubMed]
Lee TWR, Brownlee KG, Conway SP, et al. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J Cyst Fibros 2003; 2: 29-34. [PubMed]
Lee TW, Brownlee KG, Denton M, et al. Reduction in prevalence in Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatr Pulmonol 2004; 37: 104-110. [PubMed]
Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005; 293: 581-588. [PubMed]
Littlewood JM, Miller MG, Ghoneim AT, et al. Nebulised colomycin for early pseudomonas colonisation in cystic fibrosis. Lancet 1985; i: 865. [PubMed]
Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 2002; 15: 194-222. [PubMed]
Nadesalingam K, Conway SP, Denton M. Risk factors for acquisition of methicillin-resistant Staphylococcus aureus (MRSA) by patients with cystic fibrosis. J Cyst Fibros 2005; 4: 49-52. [PubMed]
Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 2001; 348: 983-984. [PubMed]
Ring E, Eber E, Erwa W, et al. Urinary N-acetyl-beta-D-glucosaminidase in patients with cystic fibrosis on long term gentamicin inhalation. Arch Dis Child 1998; 78: 540-543. [PubMed]
Rosenfeld M, Gibson RL, McNamara S, et al. Early pulmonary infection, inflammation and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 2001; 32: 356-366. [PubMed]
Steinkamp G, Tummler B, Malottke R, et al. Treatment of Pseudomonas aeruginosa colonisation in cystic fibrosis. Arch Dis Child 1989; 64: 1022-1028. [PubMed]
Taccetti G, Campana S, Festini F, et al. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J 2005; 26: 458-461.[PubMed]
Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa infection in cystic fibrosis by early treatment. Lancet 1991; 338: 725-726. [PubMed]
Vazquez C, Municio M, Corera M, et al. Early treatment of Pseudomonas aeruginosa colonisation in cystic fibrosis. Acta Paediatrica 1993; 82: 308–309.[PubMed]
Venaille TJ, Ryan G, Robinson BW. Epithelial cell damage is induced by neutrophil-derived, not pseudomonas-derived, proteases in cystic fibrosis sputum. Respir Med 1998; 92: 233-240. [PubMed]
