Renal Disease
Introduction
Renal disease is not usually considered a major problem in CF. This is surprising since the cystic fibrosis transmembrane conductance regulator (CFTR) protein is expressed in all nephron segments of the kidney (Morales et al, 2000) and both in-vitro and in-vivo studies in CF transgenic mice demonstrate abnormalities in renal ion transport (Devuyst & Guggino, 2002; Barriere et al, 2004). Mutations in the CFTR gene are not associated with major changes in renal function and patients with CF do not have structural abnormalities of the genitourinary tract, nor problems managing renal solutes. However, drug excretion by the kidneys and their ability to concentrate and dilute the urine and to excrete a salt load are altered (Stanton, 1997). It is not clear if these changes are secondary to the reduction in extra cellular fluid volume caused by excessive losses of sodium chloride in sweat and faeces or if they are related to primary defects in renal function caused by mutations in the CFTR gene.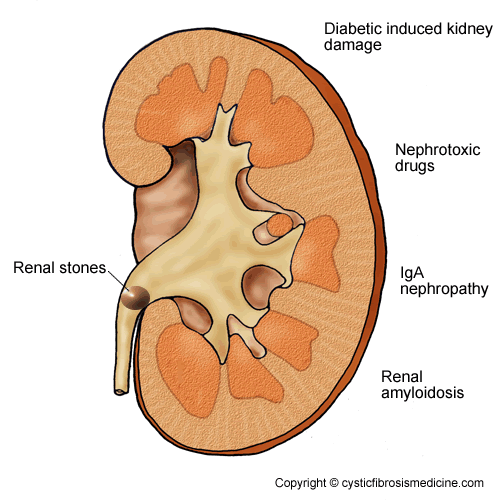
Renal stones
Various renal pathologies have been described in CF; the most clinically significant are nephrocalcinosis (calcium deposits in the kidney) and nephrolithiasis (stones in the kidney). In an autopsy study of 38 patients, microscopic nephrocalcinosis was found in 35, including two neonates and one still-born infant (Katz et al, 1988). The incidence of nephrolithiasis in CF ranges from 3% to 6.3% (Strandvik & Hjelte, 1993; Chidekel & Dolan, 1996; Matthews et al, 1996; Hoppe et al, 1998; Perez-Brayfield et al, 2002; Gibney & Goldfarb, 2003), compared to 1% to 2% in age-matched controls without CF (Gibney & Goldfarb, 2003).
The majority of renal stones are composed of calcium oxalate. In a study of 13 patients with CF and renal stones, patients presented at a mean age of 27 years with flank pain as the presenting symptom in 69% of cases (Perez-Brayfield et al, 2002).
Risk factors for renal stones
The reasons for an increased risk of stone formation in CF are multi-factorial (Matthews et al, 1996; Gutknecht, 2001) e.g. a low urine volume, hypercalciuria and hypocitraturia (Von der Heiden et al, 2003), hyperoxaluria, hyperuricosuria and increased urinary saturation of calcium oxalate (Turner et al, 2000, Perez-Brayfield et al, 2002, Terribile et al, 2006). The data are conflicting with regard to calcium and urate excretion. Some authors report that hypocalciuria is protective against nephrolithiasis in patients with CF (Bohles & Michalk, 1982) and suggest that calcium supplements, given for example for CF associated low bone mineral density, may increase the risk of stone formation. A greater degree of consensus exists for hyperoxaluria and hypocitraturia as risk factors for renal stones. Hyperoxaluria is often intestinal in origin and is associated with pancreatic insufficiency and fatty acid malabsorption (Hoppe et al, 2005). It is aggravated by the reduction or disappearance of oxalate degrading bacteria in the gut, especially the bacterium Oxalobacter formigenes (Sidhu et al, 1998). In the latter study 71% of healthy volunteers were colonised with O. formigenes compared to only 16% of patients with CF. The intensive use of antibiotics in CF care may induce permanent decolonisation with O. formigenes, increased absorption of oxalate and subsequent hyperoxaluria.
Treatment of kidney stones
Metabolic evaluation is indicated for patients with CF and urolithiasis (stones in the urinary system), as correction of risk factors may decrease stone recurrence. Pancreatic enzyme dosage should be reviewed by a specialist dietitian, fluid intake increased and supplemental citrate prescribed for patients with a proven history of nephrolithiasis. Stones may be passed in the urine without medical intervention or require extracorporeal shock wave lithotripsy (breaking the stone into fragments with sound waves) or ureteroscopy with stone extraction (Perez-Brayfield et al, 2002).
Other renal problems
Other renal pathologies have been described in CF (Stephens & Rigden, 2002):
IgA nephropathy, although rare, is the most frequently reported glomerulonephritis in patients with CF (Melzi et al, 1991), and may present with haematuria, proteinuria (blood or protein in the urine) or reduced renal function (Stirati et al, 1999).
Renal amyloidosis (abnormal protein deposits in the kidneys) is increasingly recognised as a serious complication of CF (McGlennan et al, 1986; McLaughlin et al, 2006). The majority of reported cases (15/19) have died within one year of the diagnosis of amyloid, indicating that it is a poor prognostic factor.
CF related diabetes (CFRD) will develop in more than 30% of adult patients. Although there is probably a lower prevalence of renal complications in patients with CFRD compared to non-CF patients with other forms of diabetes (Schwarzenberg et al, 2007), about 30-50% of these patients will progress to diabetic nephropathy (diabetic induced kidney damage) within five to ten years of diagnosis (Magryta et al, 1999).
Lung transplantation predisposes patients with CF to renal disease (Schindler et al, 2001). There is an accelerated loss in the glomerular filtration rate or GFR (the ability of the kidney to filter the blood and produce urine) compared to lung transplant for other pulmonary diseases (Broekroelofs et al, 2000; Hmiel et al, 2005). This post operative decline in renal function suggests that the patients renal reserve may be impaired pre-surgery. The patients are at risk of a further fall in renal function from the nephrotoxic effects of the post-operative immunosuppressive drug regimen. As post transplant survival continues to improve, renal physicians will be faced with an increasing number of patients with renal failure requiring dialysis or kidney transplant.
Nephrotoxic drugs are the commonest cause of renal injury in patients with CF. Impaired kidney function results from repeated and often prolonged treatments with aminoglycosides and/or non-steroidal anti-inflammatory drugs (NSAIDs).
In CF there is an accelerated clearance of aminoglycosides through the kidneys so that therapeutic serum drug concentrations are not reached with standard dosing. Therefore patients with CF are prescribed higher doses to achieve therapeutic drug levels and to ensure clinical efficacy, creating a greater potential for adverse drug effects. Aminoglycosides have a narrow therapeutic range, below which they will be less effective and above which there is a danger of drug toxicity. Serum aminoglycoside levels must be carefully monitored. Toxicity results from the accumulation of drug within the lysosomes of the proximal renal tubular cells and causes acute tubular necrosis (ATN). Patients typically present with non-oliguric acute renal failure (ARF) and minimal abnormalities on urinalysis. There is a strong correlation between aminoglycoside use and falling renal function, which is potentiated by the co-administration of colistin and tobramycin, although on its own, Al-Aloul et al found that colistin did not appear to be nephrotoxic (Al-Aloul et al, 2005; Masoli et al, 2005).
Patients with CF with chronic salt depletion and/or dehydration are at increased risk of NSAID induced renal injury (Bennett, 1997; Desmazes-Dufeu et al, 2005). NSAIDs inhibit the enzyme cyclo-oxygenase, blocking the production of vasodilator prostaglandins and causing renal vasoconstriction and acute renal dysfunction.
Nephrotoxic drugs may cause ARF in patients with CF at any age (Kovesi et al; 1998, Stephens & Rigden, 2001; Scott et al, 2001; Drew et al, 2002; Drew et al, 2003; Kennedy et al, 2005; Al-Aloul et al, 2005). The earliest report of ARF in patients with CF was published in 1998 which suggests that this is either a new complication of CF or that awareness of the condition has increased recently. The use of ciprofloxacin has also been associated with the development of ARF (Bald et al, 2001). The first report of a national survey of ARF in patients with CF (Bertenshaw et al, 2007) estimated the incidence risk at between 4.6 and 10.1 cases/10,000 CF patients/year, with an overall incidence in children of 7.5 cases/million/year and in adults of 125 cases/million/year. Median age at presentation of ARF was 9.7 years (range 0.4-31.8yrs). Eighty-eight percent of cases had received an intravenous aminoglycoside (76% gentamicin) at the time of onset of ARF or in the preceding week. Renal dialysis was required in 54% of cases with 92% showing a complete recovery.
Aminoglycosides and colistin are frequently prescribed to patients with CF. A once daily dosing regimen with tobramycin has been shown to be as effective as thrice daily dosing and may be less nephrotoxic in children (Smyth et al, 2005; Smyth & Tan, 2006). These agents should be prescribed with caution and treatment monitored carefully to ensure optimal efficacy while minimising the risk of nephrotoxicity. In view of the evidence for cumulative toxicity and long term renal impairment with both aminoglycosides and colistin, it is our policy to try and reduce the frequency of elective intravenous anti-pseudomonal antibiotic treatments. We are assessing patients attending the Leeds Unit who have been receiving two intravenous antibiotics for two weeks every three months for chronic P. aeruginosa infection to decide on the risk/benefit balance for each individual case. The Copenhagen group demonstrated a clear advantage from this treatment protocol in patients with chronic P. aeruginosa infection but with advances in care and the greater longevity now expected we are concerned that repeated courses over many years will result in more harm than good.
Subclinical renal impairment is now recognised more frequently with up to 42% of stable adults with CF showing a reduced creatinine clearance (Al-Aloul et al, 2005).
Monitoring renal function in patients with CF
Current methods of monitoring for renal injury (blood urea nitrogen {BUN}, serum creatinine) are not sufficiently sensitive to detect early changes in renal function. Toxicity is not detected until serious functional damage is present with a large reduction in nephron mass. Measured creatinine clearance (mCCL), using a carefully timed 24-hour urine collection is superior to other estimated formulae for the accurate estimate of GFR in patients with CF. Other formulae overestimate creatinine clearance and hence renal function, thus failing to identify the patients at increased risk (Al-Aloul et al, 2007). The need, however, for timed and accurate urine collections can lead to compliance errors in adults, and in children may not be possible. In addition, the GFR may be underestimated when muscle creatinine output is reduced e.g. malnutrition and low muscle mass.
The use of urinary N-acetyl-b-D-glucosaminidase (NAG) levels as a marker of renal dysfunction in adults has been recently reported from our own Unit (Etherington et al, 2007).The primary toxic effect of aminoglycosides is on the lysosomal system within the proximal tubule. NAG is a lysosomal enzyme present in high concentrations in the proximal tubular cells. Its molecular weight does not permit glomerular filtration, and thus raised urinary NAG levels reflect tubular dysfunction (Kunin et al, 1978). Our study showed significant urinary excretion of NAG following a single course of treatment with either tobramycin or colistin, with abnormal values persisting at follow-up in some patients. Patients with CFRD appeared to be more severely affected with elevated baseline and follow-up NAG levels. Cumulative damage was evident with repeated dosing. Greater exposure to nephrotoxic antibiotics, especially colistin, over a six year period, was associated with a greater degree of tubular dysfunction (Etherington et al, 2007). We thus recommend that colistin is reserved for patients with resistant P. aeruginosaor for those who are intolerant to tobramycin.
Other authors have used urinary NAG excretion as a marker of renal tubular function. In a study of 22 children with CF a single course of intravenous tobramycin produced a significant rise in urinary NAG excretion which was only partially reversible four weeks following discontinuation of tobramycin (Glass et al, 2005). Longitudinal NAG measurements may thus be useful in patients with CF, especially those with CFRD to identify patients at risk of developing renal impairment.
Key points• The CFTR protein is expressed in all nephron segments of the kidney• Patients are at increased risk of renal stones• A number of drugs commonly prescribed in CF care are toxic to the kidney and may provoke renal failure• Patients are at risk of subclinical renal impairment• Post-transplant patients are at increased risk of impaired renal function and this may be exacerbated by immunosuppressive therapy• The best protocols for monitoring renal function, especially in high risk patients, need to be defined• Aminoglycoside levels should be measured within the first one to two days of treatment (depending on the dosing regimen), on the eighth day and at least weekly thereafterReferencesAl-Aloul M, Miller H, Alapati S, et al. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol 2005; 39: 15-20. [PubMed]Al-Aloul M, Miller H, Stockton P, et al. Acute renal failure in CF patients chronically infected by the Liverpool epidemic Pseudomonas aeruginosa strain (LES). J Cyst Fibros 2005; 4: 197-201. [PubMed]Al-Aloul M, Jackson M, Bell G, et al. Comparison of methods of assessment of renal function in cystic fibrosis patients. J Cyst Fibros 2007; 6: 41-47. [PubMed]Bald M, Ratjen F, Nikolaizik W. Ciprofloxacin-induced acute renal failure in a patient with cystic fibrosis. Pediatr Infect Dis J. 2001; 20: 320-321. [PubMed]Barriere H, Tauc M, Poujeol P. Use of knock-out mouse models for the study of renal ion channels. J Membr Biol 2004; 198: 113-124. [PubMed]Bennett WM. Drug interactions and consequences of sodium restriction. Am J Clin Nutr 1997; 65 (Suppl): 678-681. [PubMed]Bertenshaw C, Watson AR, Lewis S, et al. Survey of acute renal failure in patients with cystic fibrosis in the United Kingdom. Thorax 2007; 62: 541-545. [PubMed]Bohles H, Michalk D. Is there a risk for kidney stone formation in cystic fibrosis? Helv Paediatrica Acta 1982; 37: 267. 7118558 [PubMed]Broekroelofs J, Navis GJ, Stegeman CA, et al. Long-term renal outcome after lung transplantation is predicted by the 1-month postoperative renal function loss. Transplantation 2000; 69: 1624-1628. [PubMed]Chidekel AS, Dolan TF Jr. Cystic fibrosis and calcium oxalate nephrolithiasis. Yale J Biol Med 1996; 69: 317-321. [PubMed]Devuyst O, Guggino WB. Chloride channels in the kidney: lessons learnt from knock-out animals. Am J Physiol Renal Physiol 2002; 283: F1176-F1191. [PubMed]Desmazes-Dufeu N, Hubert D, Burgel PR, et al. Severe dehydration and August 2003 heat wave in a cohort of adults with cystic fibrosis. Presse Med 2005; 34: 647-648. [PubMed]Drew J, Watson AR, Evans JH, et al. Antibiotics and acute renal failure in children with cystic fibrosis. Pediatr Perinat Drug Ther 2002; 5: 65-67.Drew J, Watson AR, Smyth A. Acute renal failure and cystic fibrosis. Arch Dis Child 2003; 88: 646. [PubMed]Etherington C, Bosomworth M, Clifton I, et al. Measurement of N-acetyl-b-D-glucosaminidase in adult patients with cystic fibrosis: before, during and after treatment with intravenous antibiotics. J Cyst Fibros 2007; 6: 67-73. [PubMed]Gibney EM, Goldfarb DS. The association of nephrolithiasis with cystic fibrosis. Am J Kidney Dis 2003; 42: 1-11. [PubMed]Glass S, Plant ND, Spencer DA. The effects of intravenous tobramycin on renal tubular function in children with cystic fibrosis. J Cyst Fibros 2005; 4: 221-225. [PubMed]Gutknecht DR. Kidney stones and cystic fibrosis. Am J Med 2001; 111: 83. [PubMed]Hmiel SP, Beck AM, de la Morena MT, et al. Progressive chronic kidney disease after paediatric lung transplantation. Am J Transplant 2005; 5: 1739-1747. [PubMed]Hoppe B, Hesse A, Bromme S, et al. Urinary excretion substances in patients with cystic fibrosis: a risk of urolithiasis. Pediatr Nephrol 1998; 12: 275-279. [PubMed]Hoppe B, von Unruth GE, Blank G, et al. Absorptive hyperoxaluria leads to an increased risk for urolothiasis or nephrocalcinosis in cystic fibrosis. Am J Kidney Dis 2005; 46: 440-445. [PubMed]Katz SM, Krueger LJ, Falkner B. Microscopic nephrocalcinosis in cystic fibrosis. N Engl J Med 1988; 319: 263-266. [PubMed]Kennedy SE, Henry RL, Rosenberg AR. Antibiotic related renal failure and cystic fibrosis. J Pediatr Child Health 2005; 41: 382-383. [PubMed]Kovesi TA, Swartz R, McDonald N. Transient renal failure due to simultaneous ibuprofen and aminoglycoside therapy in children with cystic fibrosis. N Engl J Med 1998; 64: 65-66. [PubMed]Kunin CM, Chesney RW, Craig WA, et al. Enzymuria as a marker of renal injury and disease: studies of N-acetyl-b-D- glucosaminidase in the general population and in patients with renal disease. Pediatrics 1978; 62: 751-760. [PubMed]Magryta CJ, Hennigar R, Weatherly M. Pathological case of the month: Diabetic nephropathy in cystic fibrosis. Arch Pediatr Adolesc Med 1999; 153: 1307-1308. [PubMed]Masoli M, Talbot H, Elworthy S, et al. Relationship between use of intravenous aminoglycosides and renal function in CF. J Cyst Fibros 2005; 4(Suppl 1): S58.Matthews LA, Doershuk CF, Stern RC, et al. Urolithiasis and cystic fibrosis. J Urol 1996; 155: 1563-1564. [PubMed]McGlennen RC, Burke BA, Dehner LP. Systemic amyloidosis complicating cystic fibrosis. Arch Pathol Lab Med 1986; 110: 879-888. [PubMed]McLaughlin AM, Crotty TB, Egan JJ, et al. Amyloidosis in cystic fibrosis: a case series. J Cyst Fibros 2006; 5: 59-61. [PubMed]Melzi ML, Costantini G, Giani M, et al. Severe nephropathy in three adolescents with cystic fibrosis. Arch Dis Child 1991; 66: 1444-1447. [PubMed]Morales MM, Falkenstein D, Lopes AG. The cystic fibrosis transmembrane regulator (CFTR) in the kidney. An Acad Bra G 2000; 72: 399-406. [PubMed]Perez-Brayfield MR, Caplan D, Gatti JM, et al. Metabolic risk factors for stone formation in patients with cystic fibrosis. J Urol 2002; 167: 480-484. [PubMed]Schindler R, Radke C, Paul K, et al. Renal problems after lung transplantation of cystic fibrosis patients. Nephrol Dial Transplant 2001; 16: 1324-1328. [PubMed]Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis related diabetes. Diabetes Care 2007; 30: 1056-1061. [PubMed]Scott CS, Retsch-Bogart GZ, Henry MM. Renal failure and vestibular toxicity in an adolescent with cystic fibrosis receiving gentamicin and standard dose ibuprofen. Pediatr Pulmonol 2001; 31: 314-316. [PubMed]Sidhu H, Hoppe B, Hesse A, et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet 1998; 352: 1026-1029. [PubMed]Smyth A, Tan KH, Hyman-Taylor P, et al. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis – the TOPIC study: a randomised controlled trial. Lancet 2005; 365: 573-578. [PubMed]Smyth AR, Tan KH. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev 2006; 3: CD002009. [PubMed]Stanton BA. Cystic fibrosis transmembrane conductance regulator (CFTR) and renal function. Wein Klin Wochenschr 1997; 109: 457-464. [PubMed]Stephens SE, Rigden SPA. Acute renal failure (ARF) in cystic fibrosis patients treated with ceftazidime and gentamicin in combination. Arch Dis Child 2001; 84(Suppl 1): A52.Stephens SE, Rigden SP. Cystic fibrosis and renal disease. Paediatr Respir Rev 2002; 3: 135-138. [PubMed]Stirati G, Antonelli M, Fofi C, et al. IgA nephropathy in cystic fibrosis. J Nephrol 1999; 12: 30-31. [PubMed]Strandvik B, Hjelte L. Nephrolithiasis in cystic fibrosis. Acta Paediatr. 1993; 82:306-7. [PubMed]Terribile M, Capuano M, Cangiano G, et al. Factors increasing the risk for stone formation in adult patients with cystic fibrosis. Nephrol Dial Transplant. 2006; 21: 1870-1875. [PubMed]Turner MA, Goldwater D, David TJ. Oxalate and calcium excretion in cystic fibrosis Arch Dis Child. 2000; 83: 244-247 [PubMed]Von der Heiden R, Balestra AP, Bianchetti MG, et al. Which factors account for renal stone formation in cystic fibrosis? Clin Nephrol. 2003 Mar;59(3):160-3. [PubMed]![]()
