
Nineties science
Unfortunately, in the early years following the identification of the gene, there were unrealistic expectations that gene therapy would change the pattern of clinical care within a short time. Since 1989 over 1500 different mutations of the CF gene have been described and, although gene replacement therapy was still not available in 2013, the first multidose gene therapy trial started in 2012 in the UK. More recently the development of drugs to treat specific genetic mutations such as G551D have been major advances.
However, during the Nineties there were a number of definite practical benefits of identification of the CF gene for people with CF and their families. Carrier detection, accurate antenatal diagnosis, preimplantation genetic diagnosis and the incorporation of DNA testing into many neonatal screening programmes have all been major advances that have followed the identification of the CF gene.
Although progress during the decade, following the identification of the CF gene, was not as rapid as many had hoped, there was nonetheless steady progress laying the foundations for future gene and drug therapy for the basic defect. Welsh and his colleagues were the first to achieve correction of the defective chloride channel in CF epithelial cells (Rich et al, 1990 below). It was shown that DF508 CFTR was incompletely processed due to defective intra-cellular processing or degradation (Cheng et al, 1990 below), and did not reach the cell membrane (Kartner et al, 1992 below); but when it did it functioned reasonably well. Also Welsh and colleagues discovered that growing CF cells at a reduced temperature improved expression of CFTR chloride channels (Denning et al, 1992 below).
Eventually CFTR was confirmed to be a chloride channel and not just a regulator of a chloride channel by Bear and colleagues; they reconstituted CFTR protein into artificial membranes and demonstrated cAMP/ATP-dependent channels with the same properties as those previously described for wild type CFTR (Bear et al, 1992 below).
Attempts to correlate phenotype and genotype proved less successful than at first expected, but the major influence of environmental factors, in particular the standard of medical care given, has been a major confounding factor. However, the definite correlation of so-called “mild” mutations with preservation of a significant degree of pancreatic function and better clinical condition is now well established (Kristidis et al, 1992 below). The vast majority of people homozygous for DF508 are pancreatic insufficient and many of those with an R117H mutation are pancreatic sufficient (Cystic Fibrosis Mutations Database). Also, certain mild mutations are associated with late presenting disease, often with normal pancreatic function and normal or near normal sweat electrolytes. An association of congenital bilateral absence of the vas deferens (CBAVD) in infertile men has been associated with a high incidence of CF mutations (Chillon et al, 1995 below), indeed some have two CF mutations, the most common pair being DF508/R117H – somewhat blurring the edges of the traditional CF diagnosis. More recently, a significant number of people with pancreatitis, but who do not have CF, have been found to carry one CF mutation (Shearer et al, 1998 below; Cohn et al, 1998 below).
It was important to create an animal model for CF for research to progress and in the early Nineties this was achieved by three groups – in North Carolina (Snouweart et al, 1992 below), Edinburgh (Dorin et al, 1992 below) and Cambridge (Radcliffe et al, 1993 below). Within a year the first report of successful gene transfer into the airways of a CF mouse was reported from Oxford (Hide et al, 1993 below); eventually in 2008 a pig model with CF was created (Rogers et al, 2008) although there were initial problems with survival of the animals and adverse reactions to anaesthetics.
Within three years of the first report of the CF gene, the first attempt at gene therapy into the nasal passages of people with CF was reported (Zabner et al, 1993 below). This report received considerable publicity in the popular press and was followed by further reports from a number of groups of gene transfer into animals and humans using either viral or liposome vectors.
The article “Physiological Basis of Cystic Fibrosis: A Historical Perspective” by Paul Quinton (Physiol Rev 1999; 79:S3-S22) [PubMed] was of particular help in formulating the explanatory comments below and can be recommended.
Abstracts of Nineties papers in chronological order
1990 Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, Oststedgaard L, Klinger KW, Welsh MJ, Smith AE. Expression and characterisation of the cystic fibrosis transmembrane conductance regulator. Nature 1990; 347:382-386. [PubMed] Identification of a cryptic bacterial promoter within the CFTR coding sequence led to the construction a complementary DNA in a low-copy-number plasmid, thereby avoiding the deleterious effects of CFTR expression on Escherischia coli. This cDNA was used to express CFTR both in vitro and in vivo and these data established several characteristics of the protein responsible for CF, would enable CFTR function to be studied and would provide a basis for diagnosis and therapy.
Gregory and colleagues surmised and validated that a cryptic region in exon 6 of the CFTR cDNA induced a transcript that was toxic to the host bacteria. When the promoter within the CFTR coding sequence was inactivated the bacteria were able to carry the plasmids containing the CFTR cDNA. Paul Quinton notes that this step was fundamental to future experiments developing cell lines for expressing the gene and its mutants.
1990 Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, Klinger KW, Smith AE, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990; 347:358-363. [PubMed]

Fig. 1 Michael Welsh medicine.uiowa.edu
The CF transmembrane conductance regulator (CFTR) was expressed in cultured CF airway epithelial cells and Cl-channel activation was assessed in single cells using a fluorescence microscopic assay and the patch-clamp technique. Expression of CFTR, but not of a mutant form of CFTR (delta F508), corrected the Cl- channel defect. Correction of the phenotypic defect demonstrated a causal relationship between mutations in the CFTR gene and defective Cl- transport which is the hallmark of the disease.
Michael Welsh (fig. 1) is Professor of Medicine and Molecular Physiology and Biophysics at the Carver College of Medicine, Iowa and Investigator at the Howard Hughes Medical Institute. He and his co-workers were the first to correct CF cells ex vivo by transfecting a CF cell line with normal DNA.
1990 Drumm ML, Pope HA, Cliff WH, Rommens JM, Marvin SA, Tsui L, Collins FS, Frizzell RA, Wilson JM. Correction of cystic fibrosis defect by retrovirus-mediated gene transfer. Cell 1990; 62:1227-1233.[PubMed]

Fig 2. Mitchell Drumm
Retrovirus-mediated gene transfer was used to demonstrate complementation of the CF defect in vitro. Retroviruses were used to transduce a functional cystic fibrosis transmembrane conductance regulator (CFTR) cDNA into a cell line derived from a patient with CF that expressed the chloride transport abnormalities characteristic of cystic fibrosis. Whole-cell patch-clamp performed on three responding clones showed that the anion efflux responses observed were due to cAMP stimulation of Cl conductance.
These findings indicated, for the first time, that the introduction of a single copy of the normal CFTR cDNA into CF cells restored the normal cAMP dependent chloride channel function.
Mitch Drumm (fig. 2), after working in Francis Collins laboratory on the identification of the CF gene, is now Professor of Genetics and Genome Sciences, Case Western Reserve University, Cleveland Ohio.
1990 Cheng SH, Gregory RJ, Marshall J, Paul D, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990; 63:827-834. [PubMed]

Fig. 3 Seng H Cheng ResearchGate
Mutations were introduced into CFTR at residues known to be altered in CF chromosomes and in residues believed to play a role in its function. Examination of the various mutant proteins indicated that normal CFTR was absent from cells containing DF508, delta 1507, K464M, F508R, and S5491 cDNA plasmids. Instead, an abnormal version of the protein was detected. The authors proposed that the mutant versions of CFTR are recognized as abnormal and remain incompletely processed in the endoplasmic reticulum of the cell where they are subsequently degraded.
Mutations with this phenotype represent at least 70% of known CF chromosomes; they suggested that the molecular basis of most CF is the absence of mature CFTR at the correct cellular location. CFTR failed to reach the cell membrane but was retained within the cell and degraded in the endoplasmic reticulum; this is a characteristic of DF 508 CFTR.
Dr Seng H Cheng is with the Genzyme Corporation, Framingham, Massachusetts at t.
1992 Kartner N, Augustinas O, Jensen TJ, Naismith Al, Riordan JR. Mislocalization of DF508 CFTR in cystic fibrosis sweat gland. Nat Genius et 1992; 1:321-327. [PubMed]
The authors note that misprocessing and mislocalization CFTR has been described for the major CF-causing mutation (DF508) in vitro. They generated monoclonal antibodies to CFTR with the aim of localizing the protein and its CF variants in vivo. Of the tissues where CFTR was observed, only the sweat gland is readily available and does not undergo secondary changes due to CF disease pathology. Sweat ducts from CF patients homozygous for DF508 did not show the typical apical membrane staining seen in control biopsies.
This demonstrates that the biosynthetic arrest and intracellular retention of DF508 CFTR, initially observed in vitro (Cheng et al, 1990 above) does occur in vivo; also that the failure to reach the cell membrane, demonstrated in vitro by Kartner et al (1992 below), also occurs in vivo.
1991 Drumm ML, Wilkinson DJ, Smit LS, Worrell RT, Strong TV, Frizzell RA, Dawson DC, Collins FS. Chloride conductance expressed by df508 and other mutant CFTRs in Xenopus oocytes. Science 1991; 254:1797-1799. [PubMed]
The cystic fibrosis transmembrane conductance regulator (CFTR) is associated with expression of a chloride conductance that is defective in cystic fibrosis. Xenopus oocytes injected with RNA coding for CFTR that contained mutations in the first nucleotide binding fold (NBF1) expressed chloride currents in response to raising adenosine 3′,5′-monophosphate (cAMP) with forskolin and 3-isobutyl-1-methylxanthine (IBMX). The mutant CFTRs were less sensitive than wild-type CFTR to this activating stimulus, and the reduction in sensitivity correlated with the severity of CF in patients carrying the corresponding mutations.
This demonstration provided the basis for detailed analyses of NBF1 function and suggested potential pharmacologic treatments for cystic fibrosis. So when mutant CFTR was sited in the cell membrane it exhibited reasonably good function in expressing chloride currents.
1991 Hardcastle J, Hardcastle PT, Taylor CJ, Goldhill J. Failure of cholinergic stimulation to induce a secretory response from the rectal mucosa in cystic fibrosis. Gut 1991; 32:1035-1039.[PubMed]

Fig.4 Chris Taylor with the Hardcastles
One of a number of studies from Sheffield on the scientific and clinical aspects of the gut in cystic fibrosis. The secretory response to cholinergic stimulation failed in rectal biopsy specimens from 5 children with CF compared with controls.
Thus, the failure of chloride secretion in the small intestine observed by this group (Taylor CJ et al. 1987 above; Taylor CJ et al, 1988 above) was also present in the rectal mucosa of people with cystic fibrosis.

Fig 5. resentation of Panchaud Medal to Professor Bob Williamson By HRH Princess Alexandra. CF Trust
1991 Professor Bob Williamson is presented with a Panchaud Medal of the UK CF Trust by HRH Princess Alexandra, Patron of the charity, for the part he and his team at St Mary’s Hospital, London had played in the identification of the CF gene over the past 15 years.
Professor Bob Williamson (fig. 5) became Professor of Molecular Genetics at St Mary’s Hospital Medical School, Imperial College London, in 1976. He worked there until 1995 when he moved to Australia as Director of the Murdoch Institute and Professor of Medical Genetics at the University of Melbourne. He not only played a major part in the research which led to the discovery of the CF gene in 1989, but also was a major supporter of the CF Trust, his optimistic dynamic attitude brought hope to all concerned with CF at the time and a belief that eventually a cure would be found. This had a major influence on the morale of the CF community.
Sir Robert Johnson, a founder Trustee of the UK CF Trust, recalls that in the late 1970s Professors Bob Williamson of St Mary’s and Alan Cuthbert of Cambridge were invited to attend a meeting of the CF Trust’s Research and Medical Advisory Committee to comment, as independent experts, on the current research efforts of the charity. Fortunately for the CF Trust, this resulted in both these distinguished scientists subsequently becoming heavily involved in and making major contributions to CF research!
1991 Ward CL, Krouse ME, Gruenert DC, Kopito RR, Wine JJ . Cystic fibrosis gene expression is not correlated with rectifying Cl- channels. Proc Natl Acad Sci U S A 1991; 88: 5277-81. [PubMed]

Fig. 6 Jeffrey Wine. profiles.stanford.edu
Cystic fibrosis involves a profound reduction of Cl- permeability in several exocrine tissues. A distinctive, outwardly rectifying, depolarization-induced Cl- channel (ORDIC channel) has been proposed to account for the Cl- conductance that is defective in CF. The recently identified CF gene is predicted to code for a 1480-amino acid integral membrane protein termed the CF transmembrane conductance regulator (CFTR). The CFTR shares sequence similarity with a superfamily of ATP- binding membrane transport proteins such as P-glycoprotein and STE6, but it also has features consistent with an ion channel function. It has been proposed that the CFTR might be an ORDIC channel.
To determine if CFTR and ORDIC channel expression are correlated, the authors surveyed various cell lines for natural variation in CFTR and ORDIC channel expression. In four human epithelial cell lines (T84, CaCo2, PANC-1, and 9HTEo-/S) that encompass the full observed range of CFTR mRNA levels and ORDIC channel density they found no correlation.
Dr. Jeffrey Wine (fig. 6) is Director of the Cystic Fibrosis Research Laboratory at Stanford. We discovered that a specific kind of sweating is rate-limited by CFTR–the anion channel product of the CF gene.
1991 Wine JJ, Brayden DJ, Hagiwara G, Krouse ME, Law TC, Muller UJ, Solc CK, Ward CL, Widdicombe JH, Xia Y. Cystic fibrosis, the CFTR, and rectifying Cl- channels. Adv Exp Med Biol 1991; 290: 253-269. [PubMed] A 25-80 pS, rectifying Cl- channel has been targeted as the exclusive or primary channel affected in CF. However, the authors found no evidence for significant activation or spontaneous activity of this channel in cell- attached patches of normal lymphoblasts or dog tracheal cells. However, in dog tracheal cells, they found lower conductance, linear Cl- channels that are spontaneously active in unstimulated cells and may show increased activity in stimulated cells. Attempts to correlate the expression of mRNA for the CFTR protein in various types of cells with the presence of the rectifying Cl- channel show a lack of correlation: i.e., depolarization-activated rectifying Cl- channels have been found in excised, inside-out patches from all cell types that they have examined to date, but the CFTR mRNA has so far only been detected in a subset of epithelial cells.
1991 Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its ion selectivity. Science 1991; 253:202-205. [PubMed]

Fig. 7 Matthew P Anderson
Beth Isreal Lahey Health
Expression of the cystic fibrosis transmembrane conductance regulator (CFTR) generates adenosine 3′,5′-monophosphate (cAMP)-regulated chloride channels, indicating that CFTR is either a chloride channel or a chloride channel regulator. To distinguish between these possibilities, basic amino acids in the putative transmembrane domains were mutated. The sequence of anion selectivity of cAMP-regulated channels in cells containing either endogenous or recombinant CFTR was bromide greater than chloride greater than iodide greater than fluoride. Mutation of the lysines at positions 95 or 335 to acidic amino acids converted the selectivity sequence to iodide greater than bromide greater than chloride greater than fluoride.
Apparently these data indicate that CFTR is a cAMP-regulated chloride channel and that lysines 95 and 335 determine anion selectivity. This was the first firm evidence indicating that CFTR was a chloride channel rather than a regulator of a chloride channel – final proof came from Bear et al, 1992 (below).
Dr Matthew P Anderson (Fig. 7) is an Associate Professor and Principal Investigator in the Departments of Pathology and Neurology and Director of Neuropathology at Beth Israel Deaconess Medical Center, an affiliate of Harvard Medical School. The Anderson Laboratory studies the molecular, cellular and neural network mechanisms responsible for disorders of membrane excitability and synaptic transmission in the central nervous system.
1992 Bear CE, Li C, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, Riordan JR. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 1992; 68:809-818.
To test the postulate that CFTR is a regulated low-conductance Cl- channel, the authors purified to homogeneity a recombinant CFTR protein from a high-level baculovirus-infected insect cell line. Upon incorporation, purified CFTR exhibited regulated chloride channel activity, providing evidence that the protein itself is the channel.

Fig 8. Christine Bear. biochemistry.utoronto.ca
This study (by reconstituting CFTR protein into artificial membranes and demonstrating cAMP/ATP-dependent channels with the same properties as those previously described for CFTR wild type expressed in vivo in patch clamping) provided final proof that CFTR was a chloride channel rather than a regulator of a chloride channel.[PubMed]
Dr Christine Bear (fig. 8) is a Professor in the Department of Biochemistry, University of Toronto. She has a special interest in epithelial chloride channels and their role in cell physiology and pathophysiology.
1992 Li C, Ramjeesingh M, Reyes E, Jensen T, Chang X, Rommens JM, Bear CE. The cystic fibrosis mutation (delta 508) does not influence the chloride activity of CFTR. Nat Genet 1993; 3:311-316. [PubMed]
The cystic fibrosis transmembrane conductance regulator (CFTR) is a phosphorylation-regulated Cl-channel. In most mammalian cells, the functional consequences of the most common CF mutation, dF508-CFTR, cannot be assessed as the mutant protein undergoes biosynthetic arrest within the cell. However, function can be studied in the baculovirus-insect cell expression system where dF508-CFTR does not appear to undergo such arrest. These results show that the phosphorylation-regulated Cl- channel activity of dF508-CFTR is similar to that of wild-type CFTR.
This observation was confirmed in comparative studies of purified dF508-CFTR and CFTR reconstituted in planar lipid bilayers. Therefore the authors suggested, as had Drumm et al, (1991), that the common dF508 mutation does not result in a significant alteration in CFTR function provided it can reach the cell membrane.
1992 Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis conductance regulator is temperature sensitive. Nature 1992; 358:761-764. [PubMed]

Fig. 9 Gerene Denning. medicine.uiowa.edu
Chloride channel activity was detected, when CFTR dF508 was expressed in Xenopus oocytes, Vero cells and Sf9 insect cells. Because the oocytes and Sf9 cells are typically maintained at lower temperatures than mammalian cells, and because processing of newly formed proteins can be sensitive to temperature, these authors tested the effect of temperature on the processing of CFTR dF508. They showed that the processing of CFTR dF508 reverts towards that of wild-type as the incubation temperature is reduced. When the processing defect is corrected, cAMP-regulated Cl- channels appear in the plasma membrane and suggest that the mutant (dF508) most commonly associated with CF is temperature-sensitive. Correcting the defect would require enhancing expression and delivery of the mutant protein to the membrane. Doubts as to whether CFTR was a chloride channel or a regulator of a channel had been resolved by Welsh’s team (Anderson et al, 1991 above).
The ability to change the function of mutant CFTR by altering the temperature led to a search for other means of rescuing CFTR function.
Dr Gerene Denning (Fig. 9) is at the Howard Hughes Medical Institute, University of Iowa College of Medicine, Iowa City
1992 Snouweart JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science 1992; 257:1083-1088. (August 1992). [PubMed]

Fig. 10 Oliver Smithies. Wikipedia
The authors generated a mouse line in which the cystic fibrosis transmembrane conductance regulator (CFTR) gene was mutated by gene targeting. Like patients with CF, mice lacking a functional CFTR gene (CFTR (-/-) mice) showed increased numbers of goblet cells and obstruction of glands with inspissated eosinophilic secretions. However, the most severe pathological changes in these mice were confined to the intestinal tract and gallbladder; there were only minor pathological alterations in the lungs and upper airways of the CFTR (-/-) mice. Possible explanations for the apparent lack of respiratory disease are the young age at which the animals were examined and the pathogen-free environment in which they were housed i.e. there had not been time to develop respiratory problems.
The production of a “CF Mouse” was a major advance. Oliver Smithies (fig. 10) (1925-2017) was awarded a share of the Nobel Prize in 2007 for this work. The award was shared with Professor Martin Evans (Ratcliff et al, 1993 below). It is worth recalling that, in common with the CF mouse, the lungs of CF infants who die in the newborn period also show little in the way of histological abnormality.
1992 Dorin JR, Dickinson P, Alton EWFW, Smith SN, Geddes DM, Stevenson BJ, et al. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature 1992; 359:211-215. (Sept 1992). [PubMed]

Fig 11. Julia Dorin
To make this mouse the cystic fibrosis transmembrane conductance regulator gene was disrupted in embryonal stem cells using an insertional gene targeting vector. Germ-line chimaeras were derived and the offspring of heterozygous crosses studied. These homozygous mutant mice survived beyond weaning and in vivo electro physiology demonstrates the predicted defect in chloride ion transport and can distinguish between each genotype. Also histological analysis showed important hallmarks of human disease pathology, including abnormalities of the colon, lung and vas deferens.
Julia Dorin’s (fig.11) was one of the three groups to create a CF mouse model – the “Edinburgh mouse”, created by insertional mouse mutation. It provided a valid model system for the development and testing of therapies for cystic fibrosis patients and was a major advance. Subsequently Julia Dorin was awarded a Personal Chair of Genetics of Host Defence in Edinburgh.
1993 Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, et al. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nature Genet 1993; 4:35-41. [PubMed]
The authors used gene targeting in embryonic stem cells to introduce an HPRT mini-gene into the coding sequence of the murine cystic fibrosis gene (cftr). This insertion introduces a termination codon in frame with the cftr coding sequence to terminate prematurely the CFTR protein within the first nucleotide binding domain. Animals homozygous for the cftr disruption fail to thrive and display a range of symptoms including meconium ileus, distal intestinal obstructions, gastrointestinal mucus accumulation and blockage of pancreatic ducts. The animals also show lacrimal gland pathology. Tracheal and caecal transepithelial current measurements demonstrate the lack of a cAMP and can be used to study gene therapy strategies.

Fig 12. Sir Martin Evans
This and the two preceding papers (Snouweart JN et al, 1992 above; Dorin et al, 1992 above) describing the creation of three types of CF mice were an essential development for further research into correction of the basic genetic defect.
Sir Martin Evans (fig. 12) first of Cambridge and then Cardiff, was awarded a share of the Nobel Prize in 2007 for his work on knockout mice – he shared the prize with Mario Capechi of Utah and Oliver Smithies of North Carolina. The Nobel citation describes the trio’s discovery for introducing specific gene modifications in mice by the use of embryonic stem cells. Martin Evans was the first to grow stem cells from early mouse embryos. He injected stem cells from one strain into the embryos of another strain then implanted the embryo into a surrogate mother leading to mosaic-celled mice.
1993 Hyde SC, Gill DR, Higgins CF, Tresize AEO, MacVinish LJ, Cuthbert AW, Ratcliff R, Evans MJ, Colledge WH. Correction of ion transport defect in cystic fibrosis transgenic mice by gene therapy. Nature 1993; 362:250-255. [PubMed]
Steve Hyde and his wife Deborah Gill (fig. 13) and their colleagues in Oxford and Cambridge were the first to demonstrate the use of liposomes as a vector to deliver a CFTR expression plasmid to epithelia of the airway and to alveoli deep in the lungs of CF mice (cf/cf), leading to the correction of the ion conductance defects found in the trachea.
Steve Hyde (Fig. 13) became Co-Director of the Gene Medicine Research Group and Professor of Molecular Therapy, Oxford University.
Deborah Gill (Fig. 13) became Co-director of the Gene Medicine Research Group and Professor of Gene Medicine, Oxford University.

Fig 13. Deborah Gill and Steve Hyde. Author’s photo
This was the first correction of the basic defect in CF mice and illustrated the eventual feasibility of gene therapy for the pulmonary aspects of CF in humans. Hopes were high at this stage for the development of gene therapy within a relatively short time. These findings were confirmed in a similar study by Eric Alton and colleagues from Imperial College, London (Alton EW et al. Nat Genet 1993; 5:135-142). However, it was 20 years later (2013) before the first multi-dose gene therapy trial was underway in the UK.
1993 Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE, Welsh MJ. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993; 75:207-216.[PubMed]

Fig 14. Joe Zabner. medicine.uiowa.edu
This was the first study to evaluate the potential of direct transfer of cystic fibrosis transmembrane conductance regulator (CFTR) cDNA for the treatment of people with cystic fibrosis. The authors administered an E1-deficient adenovirus, encoding CFTR, to a defined area of nasal airway epithelium of three adults with cystic fibrosis. This treatment corrected the Cl- transport defect that is characteristic of CF-affected epithelia. After treatment, there was a decrease in the abnormally elevated basal transepithelial voltage, and the normal response to a cAMP agonist was restored.
At the time this treatment was approached with considerable caution as it was a “first” in humans but fortunately there was no evidence of viral replication or virus-associated adverse effects. These were the first nasal studies in humans attempting to correct the CF defect using adenoviral vectors. These data were considered to represent a small step in achieving long-term improvement of CF lung function by gene therapy but, quite understandably, received a great deal of media publicity at the time.
Joseph Zabner (Fig. 14) is Professor of Internal Medicine – Pulmonary, Critical Care and Occupational MedicineCarver College of Medicine Iowa.
Other studies of gene therapy followed using viral vectors to the lungs (Crystal et al, 1994 below), and nose (Knowles et al, 1995 below) and using liposomal vectors to the nose (Caplan et al, 1995 below; Porteous et al, 1997 below; Gill et al, 1997 below) to the nose and lungs (Alton et al, 1999) and repeated doses using liposomes to the nose (Hyde et al, 2000 below). It is salutary that gene therapy for CF was still a considerable way from the clinic although the UK multidose gene therapy trial started in 2013. Prof. Joseph Zabner (fig. 9) led the team who performed this, the first gene transfer in patients with cystic fibrosis.
1994 Gabriel SE, Brigman KN, Koller BH, Boucher RC, Jackson Stutts M. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 1994; 266:107-109. [PubMed]
This study sought an explanation for the high frequency of the CF gene in the population (1 in 25). It had been suggested that in the past that CF heterozygotes had an increased resistance to cholera – so-called “heterozygote advantage”. Heterozygotes expressed only 50% of the normal amount of CFTR protein in the intestinal epithelium and secreted 50% of the normal fluid and chloride ion in response to cholera toxin – perhaps potentially lessening the effects of an attack of cholera in years past.
These findings supported the suggestion that, over many generations, CF heterozygotes might possess a selective advantage in their reduced response to cholera toxin resulting in a survival advantage if they contracted cholera or other severe diarrhoeal diseases.
Dr S E Gabriel îs în the Department of Medicine, North Carolina State University.
1994 Crystal RG, McElvaney NG, Rosenfeld MA, Chu CS, Mastrangeli A, Hay JG, Brody SL, Jaffe HA, Eissa NT, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet 1994; 8:42-51.[PubMed]
This first human gene therapy trial involving the lungs was reported in the press in April 1993. A recombinant adenovirus vector (AdCFTR) containing the normal human CFTR cDNA was administered to the nasal and bronchial epithelium of four individuals with cystic fibrosis.

Fig 15. Ron Crystal
Follow-up at six to 12 months demonstrated no long term adverse effects. Thus, it was considered feasible to use an adenovirus vector to transfer and express the CFTR cDNA in the respiratory epithelium of individuals with CF. It was suggested that correction of the CF phenotype of the airway epithelium might be achieved with this strategy. Unfortunately it soon became apparent that viral vectors were not suitable for repeated administration as they caused an increasing antibody response.
Ron Crystal (fig. 15) of New York was one of the early clinical gene therapy researchers and his group has been involved in many aspects of gene and stem cell therapies. He was still active in this area in 2021, launching his own company Lexeo Therapeutics, with an $85 million Series A to drive three of the company’s AAV-administered candidates to market.. Crystal will take the role of chief scientific adviser with Pfizer veteran Nolan Townsend joining as CEO.
1995 Knowles MR, Hohneker KW, Zhou Z. Olsen JC, Noah TL, Hu PC, Leigh MW, Engelhardt JF, Edwards LJ, Jones KR, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Eng J Med 1995; 333:823-831. [PubMed]
An adenoviral vector containing the normal CFTR complementary DNA in increasing doses was administered to the nasal epithelium of 12 patients with CF with no obvious beneficial effect. The authors concluded that in patients with CF, adenoviral-vector-mediated transfer of the CFTR gene did not correct functional defects in nasal epithelium, and local inflammatory responses limited the dose of adenovirus that could be administered.
Another disappointing early gene therapy trial this time from North Carolina using a viral vector. So enthusiasm for viral vectors gradually waned as it became apparent that a significant level of antibodies developed, reducing the potential for their repeated administration.
1995 Colledge WH, Abella BS, Southern KW, Ratcliff RA, Jiang C, Chen SH, MacVinish LJ, Anderson JR, Cuthbert AW, Evans MJ. Generation and characteristics of a DF508 cystic fibrosis mouse model. Nature Genet 1995; 10:445-452. [PubMed]
Fig 16. Bill College
These mice show changes characteristic of the CF phenotype, die from peritonitis and show abnormalities of Cl transport. But they produce CFTR transcripts and show the temperature dependent trafficking defect described in the human df508 CFTR protein. There is a functional CFTR Cl channel not present in null mice or at 37C but was detected at 27C; hence they are an accurate DF508 model.
Professor William Colledge (fig. 16) of Cambridge is Professor of Reproductive Physiology and one the UK’s leading scientists involved in CF. His group has employed transgenic mouse models of human disease to understand the mechanisms of disease progression and to develop new treatment strategies. This technology centres around the use of genetically manipulated embryonic stem cells to generate mice carrying specific mutations (also Radcliff et al, 1993 above).
1995 Caplen NJ, Alton EWFW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffrey K, Hodson ME, Coutelle C, Huang L, Porteous DJ, Williamson R, Geddes DM. Liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med 1995; 1:39-46. [PubMed]

Fig 18 Natasha Caplan Center for cancer Research

Fig. 17.Eric Alton
The first UK nasal gene therapy study using liposomal vectors from the Royal Brompton Hospital in London with the cooperation of other centres. A double blind placebo controlled trial in nine CF subjects receiving cationic liposome complexed with complementary DNA encoding the CF transmembrane conductance regulator and six CF subjects receiving only the liposome applied to their nasal epithelium. There were no adverse effects. A partial restoration of the deficit between CF and non-CF subjects of some 20% was seen. Plasmid DNA and Transgene derived RNA were detected in the majority of subjects.
The authors concluded that the efficiency and duration would have to improve to achieve meaningful therapeutic benefit.
Many of the contributors to this first UK gene therapy study from London and Edinburgh led by Professor Eric Alton (Fig. 17) would eventually form the UK Gene Therapy Consortium which from 2001 would be the main research focus of the UK CF Trust until 2011 and would eventually undertake a multidose gene therapy trial in the UK in 2012/14.
Natasha J Caplan (Fig. 18) is now an investigator and Head of Functional Genetics at the Center for Cancer Research, Bethesda.
1997 Porteous DJ, Dorin R, McLachlan G, Davidson-Smith H, Davidson H, Stevenson BJ, Carothers AD, Wallace WA, Moralee S, Hoenes C, Kallmeyer G, Michaelis U, Naujoks K, Ho LP, Samways JM, Imrie M, Greening AP, Innes JA. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997; 4:210-18. [PubMed]

Fig 19. David Porteous
One of the major UK gene therapy studies from Edinburgh. Prof. David Porteous’s group (figure 12) tested the safety and efficacy of gene delivery to the nasal epithelium of CF patients using pCMV-CFTR-DOTAP cationic liposome complex. A single dose of 400 micrograms pCMV-CFTR: 2.4 mg DOTAP was administered in a randomised, double-blinded fashion to the nasal epithelium of eight CF patients, with a further eight receiving buffer only. Transgene DNA was detected in seven of the eight treated patients up to 28 days after treatment and vector derived CFTR mRNA in two of the seven patients at +3 and +7 days. Transepithelial ion transport was assayed before and after treatment by nasal potential difference during drug perfusion and by SPQ fluorescence halide ion conductance.
Partial, sustained correction of CFTR-related functional changes toward normal values were detected in two of the eight treated patients. The authors concluded that results justified further studies with pCMV-CFTR-DOTAP aimed at treating CF lung disease.
This was the second UK study of gene therapy by the Edinburgh group led by David Porteous (Fig 19) who later would be one of the three principal researchers in the UK Gene Therapy Consortium when it was formed in 2001.
1997 Gill DR, Southern KW, Mofford KA, Seddon T, Huang L, Sorgi F, Thomson A, MacVinish LJ, Ratcliff R, Bilton D, Littlewood JM, Middleton PG, Colledge WH, Cuthbert AW, Evans MJ, Higgins CF, Hyde SC. A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997; 4:199-209. [PubMed]

Fig 21. Steven Hyde Radcliffe Department of Medicine

Fig 20. Deborah Gill ResearchGate
From the Oxford Group and many other UK collaborators, a double-blinded, placebo-controlled, clinical study of the transfer of the CFTR cDNA to the nasal epithelium of 12 CF patients coordinated by Dr Kevin Southern. Cationic liposomes complexed with plasmid containing the human CFTR cDNA were administered to eight patients, whilst four patients received placebo. Biopsies of the nasal epithelium taken seven days after dosing were normal. No significant changes in the clinical parameters were observed. Functional expression of CFTR assessed by in vivo nasal potential difference measurements showed transient correction of the CF chloride transport abnormality in two patients. Fluorescence microscopy demonstrated CFTR function ex vivo in cells from nasal brushings. In total, some evidence of functional CFTR gene transfer was obtained in six out of the eight treated patients.

Fig 22. Kevin Southern. Author’s photo
This gene therapy study was led by the Oxford Group, Deborah Gill (Fig. 20) and Steven Hyde (Fig. 21) that would be the third member of the UK Gene Therapy Consortium which would be formed in 2001. These results provided further proof of concept for liposome-mediated CF gene transfer which would be the vector ultimately chosen by the Consortium for further development to use in their clinical trials which eventually started in 2012.
Dr Kevin Southern (fig. 22) started his involvement with CF as CF Research Fellow at the CF Centre at St James’s in Leeds. He is now Professor of Paediatrics at Liverpool University and consultant at the Alder Hey Children’s Hospital. Kevin was awarded his PhD for the work reported here and has since developed a major role in CF research and care both in the UK and Europe.
1997 Loirat F, Hazout S, Lucotte G. G542X as a probable Phoenician cystic fibrosis mutation. Hum Biol 1997; 69:419-425.[PubMed]
When analyzed by origin, the frequency of the G542X CF mutation (the second most common CF mutation in Europe after DF508) varies between population groups in Europe being lower in north-eastern Europeans than in south-western Europeans. The more elevated values of G542X frequency correspond to ancient sites of occupation by occidental Phoenicians. N1303K as been linked to ancient Mediterranean populations and G551D associated with ancient Celtic tribes (Cashman SM et al, Hum Hered 1995; 45:6-12. [PubMed].
1998 Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998; 23;95:1005-15. [PubMed]

Fig 23. Richard Boucher
The pathogenesis of cystic fibrosis airways infection is unknown. Two hypotheses, “hypotonic [low salt]/defensin” and “isotonic volume transport/mucus clearance,” attempt to link defects in cystic fibrosis transmembrane conductance regulator-mediated ion transport to CF airways disease. The authors tested these hypotheses with planar and cylindrical culture models and found no evidence that the liquids lining airway surfaces were hypotonic or that salt concentrations differed between CF and normal cultures. In contrast, CF airway epithelia exhibited abnormally high rates of airway surface liquid absorption, which depleted the periciliary liquid layer and abolished mucus transport. The failure to clear thickened mucus from airway surfaces likely initiates CF airways infection.
These data indicate that therapy for CF lung disease should not be directed at modulation of ionic composition, but rather at restoring volume (salt and water) on airway surfaces. This study was important in developing the the low volume hypothesis relating the molecular abnormalities to the respiratory problems – a theory which received strong support from Richard Boucher (Fig. 23) over the years.
1998 Rubenstein R, Zeitlin P. A pilot clinical trial of sodium 4-phenylbutyrate (biphenyl) in DF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Med 1998; 157:484-490.[PubMed]

Fig 24. Pam Zeitlin P R Newswire
Sodium 4-phenylbutyrate (Buphenyl, 4PBA) was a new FDA approved drug for management of urea cycle disorders. The authors had previously presented in vitro data suggesting the drug induced CFTR channel function on the plasma membrane of deltaF508-expressing CF airway epithelial cells (Rubenstein, R. C., and P. L. Zeitlin, 1997. J. Clin. Invest. 100:2457-2463). Here they performed a randomized, double-blind, placebo-controlled trial in 18 dF508-homozygous patients with cystic fibrosis. Subjects in the 4PBA group demonstrated small, but statistically significant improvements of the nasal potential difference response to perfusion of an isoproterenol/amiloride/chloride-free solution – a measure reflecting epithelial CFTR function that is highly discriminatory between patients with and without cystic fibrosis. Subjects who had received the 4PBA did not demonstrate significantly reduced sweat chloride concentrations or alterations in their nasal potential differences. Side effects due to drug therapy were minimal and comparable in the two groups.
These data are consistent with 4PBA therapy inducing CFTR function in the nasal epithelia of deltaF508-homozygous CF patients. As the problems of gene replacement therapy became apparent in the USA there was increasing interest in treatment to correct and/or potentiate the function of the mutant abnormal CFTR. Already Denning et al, 1992 (above) had shown the function of mutant CFTR could be changed when the temperature was lowered which suggested the possibility of improving the function of even abnormal CFTR.
Through the Nineties improving the function of abnormal CFTR by pharmacological means became the main focus of North American research in contrast to the UK where gene therapy became the main focus of research.
Dr Pam Zeitlin (fig. 24) of Johns Hopkins and subsequently National Jewish Health is a leading N. American paediatrician who has been actively involved in various aspects of CF research.
1998 Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, Braganza J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Eng J Med 1998; 339:645-652. [PubMed]
The pancreatic lesions of cystic fibrosis develop in utero and closely resemble those of chronic pancreatitis. These authors hypothesized that mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene may be more common than expected among patients with chronic pancreatitis. Therefore 134 consecutive patients with chronic pancreatitis were examined for 22 mutations of the CFTR gene that together accounting for 95 percent of all mutations in patients with CF in North West England. The 94 male and 40 female patients ranged in age from 16 to 86 years. None had a mutation on both copies of the CFTR gene but 18 patients (13.4 percent) had a CFTR mutation on one chromosome, as compared with a frequency of 5.3 percent among 600 local unrelated partners of persons with a family history of cystic fibrosis (P<0.001). A total of 10.4 percent of the patients had the 5T allele in intron 8 (14 of 134), which is twice the expected frequency (P=0.008) (error – see below). Four patients were heterozygous for both a CFTR mutation and the 5T allele. Patients with a CFTR mutation were younger than those with no mutations. None had the clinical or laboratory features of cystic fibrosis.
The authors concluded that mutations of the CFTR gene and the 5T genotype are associated with chronic pancreatitis.

Fig 25. Martin Schwarz
This paper from Manchester UK is reported in some detail as it was the first report of a strong association between mutations in the CFTR gene and pancreatitis in people who did not have cystic fibrosis.
Martin Schwarz, Consultant Clinical Molecular Geneticist in Manchester (fig. 25) told me that the total percentage with 5T was only 5% (not 10.4% as stated in the paper) and so not significant. The abnormal CFTR genotypes in these patients with pancreatitis resemble those associated with male infertility. The findings of Cohn et al, 1998 (below) were similar to those in this study (Cohn et al, 1998 below) and appeared in the same issue of the New England Journal of Medicine.
1998 Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 1998; 339: 653-8. [PubMed]

Fig. 26 Jonathan A Cohn. Linkedin
Twenty seven adult patients with idiopathic pancreatitis were tested for 17 CFTR mutations and for the 5T allele in intron 8 of the CFTR gene. (The 5T allele reduces the level of functional CFTR and is associated with an inherited form of infertility in males). Patients with two abnormal CFTR alleles were further evaluated for unrecognized CF-related lung disease, and both base-line and CFTR- mediated ion transport were measured in the nasal mucosa. Ten patients (37%) with idiopathic chronic pancreatitis had at least one abnormal CFTR allele. Eight CFTR mutations were detected. In three patients both alleles were affected but they did not have lung disease typical of CF on the basis of sweat testing, spirometry, or base-line nasal potential-difference measurements but they did have abnormal nasal cyclic AMP-mediated chloride transport.
Both the above papers independently confirmed a definite association of pancreatitis and CF mutations and appeared on Sept 3rd 1998 in the same issue of the New England Journal of Medicine.
J A Cohn (Fig. 26) is in the Department of Medicine, Veterans Affairs and Duke University Medical Centers, Durham, NC 27710, USA.
1999 Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, Davies J, Smith SN, Browning J, Davies MG, Hodson ME, Durham SR, Li D, Jeffery PK, Scallan M, Balfour R, Eastman SJ, Cheng SH, Smith AE, Meeker D, Geddes DM. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet 1999; 353:947-54. [PubMed]
The second Brompton gene therapy study and the most important to date, reporting gene therapy both into the nose and also into the lungs showing some correction of the basic defect in both. Eight patients with CF were randomly assigned DNA-lipid complex (active) by nebulisation into the lungs followed 1 week later by administration to the nose. Eight control patients followed the same protocol but with the lipid alone (placebo). Safety was assessed clinically, by radiography, by pulmonary function, by induced sputum, and by histological analysis. Efficacy was assessed by analysis of
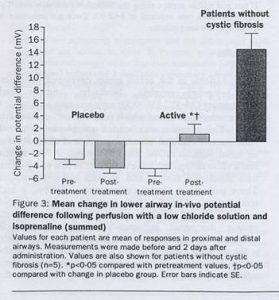
Fig 27. Potential differences
vector-specific CFTR DNA and mRNA, in-vivo potential difference, epifluorescence assay of chloride efflux

Fig 28. Eric Alton
, and bacterial adherence. Seven of the eight patients receiving the active complex reported mild influenza-like symptoms that resolved within 36 hours. Six of eight patients in both the active and placebo groups reported mild airway symptoms over a period of 12 hrs following pulmonary administration. No specific treatment was required for either event. Pulmonary administration resulted in a significant (p<0.05) degree of correction of the chloride abnormality in the patients receiving active treatment but not in those on placebo when assessed by in-vivo potential difference (figure 15) and chloride efflux. Bacterial adherence was also reduced. There were no alterations in the sodium transport abnormality. A similar pattern occurred following nasal administration.
This was the first UK study of gene therapy into the lungs of people with CF using a liposome vector and was the starting point of the UK Gene Therapy Consortium which became the main research project funded by the UK CF Trust in the new millennium under the leadership of Professor Eric Alton (figure 28) of Imperial College, London with Prof David Porteous and Dr Chris Boyd (Edinburgh) and Drs Steve Hyde and Deborah Gill (Oxford). The UK Gene Therapy Consortium eventually started a multi dose gene therapy trial in 2012/13. e>
1999 Freedman SD Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr-/-mice. PNAS 1999; 96:13995-14000.[PubMed]

Fig. 29 Steven David Freedman. eMedevents
A deficiency in essential fatty acid metabolism has been reported previously in plasma from patients with cystic fibrosis. The objective of this present study was to determine whether alterations in fatty acid metabolism were specific to CF-regulated organs and whether they played a role in the expression of disease.
A membrane lipid imbalance was found in ileum, pancreas, and lung from cftr(-/-) mice characterized by an increase in phospholipid-bound arachidonic acid and a decrease in phospholipid-bound docosahexaenoic acid (DHA). This lipid imbalance was observed in organs pathologically affected by CF including lung, pancreas, and ileum and was not secondary to impaired intestinal absorption or hepatic biosynthesis of DHA. As proof of concept, oral administration of DHA to cftr (-/-) mice corrected this lipid imbalance and reversed the observed pathological manifestations in the pancreas.
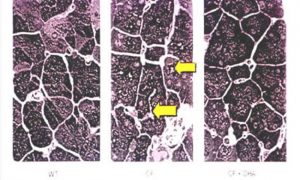
Fig 30. Pancreatic sections. Left – wild type mice. Centre cftr-/cftr mice. Right cftr-/cftr- mice on DHA.
The authors considered these results strongly suggest that certain phenotypic manifestations of CF may result from remediable alterations in phospholipid-bound arachidonic acid and DHA levels (figure 30)
Dr. Steven Freedman,(Fig. 29) is the Chief of the Division of Translational Research and Professor of Medicine at Harvard Medical School.
SUBSEQUENT PAPERS ON THIS SUBJECT ARE MENTIONED HERE
2004 Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O’Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Eng J Med 2004; 350:560-569. [PubMed]
The 1999 paper, caused considerable interest in medical circles and in the media at the 1999 North American CF Conference in Seattle, as Dr Freedman of the Beth Israel Deaconess Medical Centre Boston, suggested that essential fatty acid imbalance, affected the phenotypic expression of the CF defect and hence implied that the CF phenotype could be modified by correction of the imbalance.
This latest revival in interest in essential fatty acids was made possible by the availability of CF mice and the opportunity to examine their pancreatic tissue.
2007 Beharry S, Ackerley C, Corey M, Kent G, Heng YM, Christensen H, Luk C, Yantiss RK, Nasser IA, Zaman M, Freedman SD, Durie PR. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol – Gastr L 2007; 292:G839-48.[PubMed]

Fig. 31 Satti Beharry ResearchGate
Unfortunately subsequent studies by this group failed to confirm the fundamental importance of these findings (Beharry et al, 2007) but did suggest that DHA therapy may release endogenous inhibitors of inflammation (below) although it is fair to say that the initial enthusiasm has waned (Freedman SD et al, 2004; Beharry et al, 2007).
The authors previously demonstrated that arachidonic acid levels are increased and docosahexaenoic acid levels are decreased in affected tissues from cystic fibrosis-knockout mice (Freedman et al, 1999 above). In this present study of fatty acids from nasal- and rectal-biopsy specimens, nasal epithelial scrapings, and plasma were analyzed from 38 subjects with CF and compared with results in 13 obligate heterozygotes, 24 healthy controls, 11 subjects with inflammatory bowel disease, 9 subjects with upper respiratory tract infection, and 16 subjects with asthma. The ratio of arachidonic to docosahexaenoic acid was increased in mucosal and submucosal nasal-biopsy specimens (P<0.001) and rectal-biopsy specimens (P=0.009) from subjects with CF compared with the healthy control subjects. In nasal tissue, this change reflected an increase in arachidonic acid levels and a decrease in docosahexaenoic acid levels. In cells from nasal mucosa, the ratio of arachidonic to docosahexaenoic acid was increased in subjects with cystic fibrosis (P<0.001), as compared with healthy controls, with values in obligate heterozygotes intermediate between these two groups (P<0.001).
The authors concluded that these data indicated that alterations in fatty acids similar to those in cystic fibrosis-knockout mice are present in CFTR-expressing tissue from subjects with cystic fibrosis.
Dr Satti Behary (Fig. 31) is the laboratory research coordinator Division of Gastroenterology, hepatology and Nutrition Sick Childrens Toronto
Despite considerable published work, up to 2012, EFA therapy has not been established as beneficial in people with CF; nor has this work had a major impact on the understanding of or treatment of cystic fibrosis as was hoped when first reported in 1999.
A congenic C57Bl/6J cystic fibrosis transmembrane conductance regulator (Cftr)(-/-) mouse model, which develops cystic fibrosis (CF)-like pathology in all organs, was used to evaluate the short- and long-term therapeutic effects of dietary docosahexaenoic acid (DHA). Thirty-day-old Cftr (-/-) mice and wild-type littermates were randomized to receive a liquid diet with or without DHA (40 mg/day). Animals were killed for histological and lipid analysis after 7, 30, and 60 days of therapy. DHA had no significant therapeutic or harmful effect on the lung, pancreas, or ileum of the Cftr (-/-) mice or their wild-type littermates. In contrast, dietary DHA resulted in highly significant amelioration of the severity of liver disease in the Cftr (-/-) mice, primarily a reduction in the degree of peri-portal inflammation.
The authors concluded that inhibition of cytokines and/or eicosanoid metabolism and release of endogenous inhibitors of inflammation by the DHA may account for the anti-inflammatory effects in the liver of this congenic murine model of CF. The potential therapeutic benefits of DHA in severe CF-associated liver disease remain to be explored. So the EFA story continues even in 2013 and although dietary DHA supplements had no effect on many organs of the Cftr (-/-) mouse including the pancreas as had been suggested in 1999, there was considerably less hepatic inflammation in the treated mice.
1999 Sheppard DN, Welsh MJ. Structure and function of the cystic fibrosis transmembrane conductance regulator chloride channel. Physiol Rev 1999; 79 Supplement S23 – S45. [PubMed]

Fig. 32 David Sheppard
A useful summary of the functions of CFTR chloride channel which is a unique member of the ABC transporter family. Situated in the apical membranes of epithelia it mediates transmembrane salt and water transport. Dysfunction causes cystic fibrosis. The CFTR is composed of 5 domains: two membrane spanning domains (MSDs), two nucleotide binding domains (NBDs) and a regulatory domain (R). The structure and function of the channel is reviewed. The MSDs form the channel pore, phosphorylation of the R domain determines channel activity, ATP hydrolysis by the NBDs controls channel gating. Current knowledge of the channel’s structure may help understanding of function and dysfunction in cystic fibrosis.
David Sheppard (Fig.32) is Reader and subsequently Professor in the Department of Physiology and Pharmacology, University of Bristol. He is heavily involved in scientific research particularly relating to the structure and functions of CFTR. In 2006 David was instrumental in obtaining European Community funding for EuroCareCF, a major Europe-wide collaborative undertaking with the main object of “translating research results into patient care” and is the General Coordinator and leader of the project (www.eurocarecf.eu).
1999 Becq F, Mettey Y, Gray MA, Galietta LJ, Dormer RL, Merten M, Metaye T, Chappe V, Marvingt-Mounir C, Zegarra-Moran O, Tarran R, Bulteau L, Derand R, Pereira MM, McPherson MA, Rogier C, Joffre M, Argent BE, Sarrouilhe D, Kammouni W, Figarella C, Verrier B, Gola M, Vierfond JM. Development of substituted Benzo[c]quinolizinium compounds as novel activators of the cystic fibrosis chloride channel. J Biol Chem 1999; 274:27415-25. [PubMed]

Fig. 33 Fred Becq author’s photo
The authors synthesized a series of substituted benzo[c]quinolizinium (MPB) compounds. Among them, 6-hydroxy-7-chlorobenzo[c]quinolizinium (MPB-27) and 6-hydroxy-10-chlorobenzo[c]quinolizinium (MPB-07), were shown to be potent and selective activators of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. The results also provide evidence that substituted benzo[c]quinolizinium compounds are a novel family of activators of CFTR and of CFTR-mediated protein secretion.
Professor Fred Becq (Fig. 33) of the Institute of Cell Physiology and Biology (CNRS/Université de Poitiers) is a leading researcher into the pharmacological means of activating the abnormal chloride channels in cystic fibrosis. Later he was involved with the use of miglustat a drug already authorized for use in Gaucher’s disease.

