Nebulisers and Compressors
The Leeds Method of Management. August, 2018. Nebulisers and compressors [online]. Leeds Regional Adult and Paediatric Cystic Fibrosis Units, St James’s University Hospital, Leeds, UK. Available from www.cysticfibrosis.online
Introduction
Nebulisers deliver a fine mist of water, saline or drugs in a form that can be inhaled by the patient. If a mask is used, as advised for very young children, the mouth should be open or all the medication is deposited in the nose and none reaches the lower respiratory tract. Older patients should always use a mouthpiece and breathe through the mouth rather than the nose. An exception is patients after lung transplant who may use a mask to target specific infection in the upper airways and/or sinuses.
Inhaled therapy often plays a major role in treatment and it is of vital importance that all factors regarding nebuliser therapy are considered each time a patient is reviewed.
The correct combination of drug, nebuliser and compressor will ensure optimal droplet size, thus increasing drug deposition in areas where it is required and so improve patients’ treatment.

Figure 1. The Sidestream plus and Pari LC Plus, which are used for antibiotics and anti fungals, direct the patient’s inspiratory flow through the nebuliser chamber, thereby increasing the effective inhaled nebuliser output. For this to occur the respiratory flow must exceed the compressor flow rate through the nebuliser for a significant proportion of the inspiratory period. This does not always occur in very young children and patients with poor lung function.
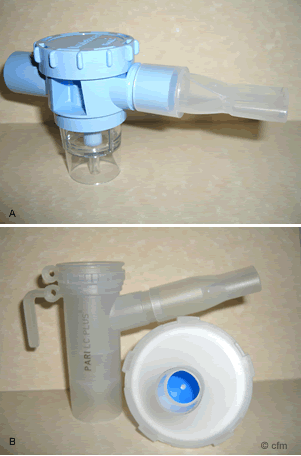
Figure 2. A) Ventstream B) Pari LC Plus
The most commonly used compressorshave a flow rate of approximately six to eight litres/minute which is adequate to drive Venturi type nebulisers. Each compressor and nebuliser has its own characteristics, so simply combining any nebuliser with any compressor may not provide the optimum performance characteristics and hence the greatest benefit to the patient.
Nebuliser systems have been developed which exploit the Venturi effect to enhance drug delivery. These systems include Sidestream® and sidestream plus from Philips Respironics, and the Pari LC Plus® from Pari.
The Sidestream, which is used for bronchodilators, steroids, and Pulmozyme®, and hypertonic saline uses the compressor airflow to entrain additional air (Venturi effect) through the nebuliser, thereby increasing both the output from the nebuliser and drug deposition, and reducing nebulisation time.
General care
It is important that the disposable nebulisers are changed regularly. Their useful life varies according to the type. Compressors should be serviced regularly by the hospital Medical Physics Department or whichever department of the hospital is responsible for supplying them.
The cleaning of nebulisers and compressors is an essential part of the treatment regimen. Studies have shown that nebulisers may be contaminated with a variety of fungi and bacteria, including Burkholderia cepacia complex (Bcc) and S. maltophilia (Hutchinson et al, 1996; Denton et al, 2003, Peckham et al, 2016). Nebulisers should be washed after every use in hot soapy water, rinsed well, dried with a paper towel and left disassembled to air dry away from the sink (Denton et al, 2003). Those paying close attention to good nebuliser care, particularly thorough drying, are least likely to suffer problems with nebuliser contamination and may reduce their risk of colonisation with multi-resistant pathogens (Walsh et al, 2002). Nebulisers should be boiled in water containing a few drops of a liquid detergent for 10 minutes once a week.
Tubing should be changed on a regular basis as this becomes damp during use and is difficult to dry. We suggest that the compressor be left switched on for a short time following disconnection of the nebuliser in order to blow out any water droplets.

Figure 3. A) I-neb® B) eFlow®
Inlet and outlet filters on the compressor should be changed every three to six months (see manufacturers recommendations) and compressors should be serviced yearly.
- Membrane/Mesh Technology
Advances in membrane/mesh technology has allowed inhaled drug delivery to be revolutionised. Particles within the respirable range (median size of 4.1-4.5 microns) are generated when the liquid passes through the membrane. Devices using this technology include the eFlow® (Pari medical), and I-neb® (Respironics). The I-neb® is also an Adaptive Aerosol Delivery (AAD) device which delivers medication during the first 50% of inspiration only and automatically stops when the pre-set dose has been delivered. The aerosol is only produced when the patient is inhaling properly through the mouthpiece. There is therefore no drug wastage if the patient is coughing, talking, laughing or nasal breathing. Both devices dramatically reduce treatment time, are portable and operate silently. The I-neb® is only available if the patient is prescribed Promixin® (ZAMBON)
It is important that the immediate effect of ANY new inhaled drug is monitored by performing respiratory function tests before and after nebulisation. Occasionally there may be deterioration in lung function due to irritation of the airways causing secondary bronchospasm. This is more likely to occur with antibiotics, e.g. colistin. If the patient feels that the nebulised treatment makes him/her worse it should be stopped immediately. It is important to observe any deterioration which occasionally occurs in infants following the administration of nebulised drugs.
Key points
• Older patients should always use a mouthpiece and breathe through their mouths when using a nebuliser
• Proper cleaning of nebulisers and compressors is an essential part of the patient’s management
• Membrane/mesh technology allows rapid drug delivery
References
Denton M, Rajgopal A, Mooney L, et al. Stenotrophomonas maltophilia contamination of nebulizers used to deliver aerosolized therapy to in patients with cystic fibrosis. J Hosp Infect 2003; 55:180-183. [PubMed]
Hutchinson GR, Parker S, Pryor JA, et al. Home-use nebulizers: a potential source of Burkholderia cepacia and other colistin-resistant, gram-negative bacteria in patients with cystic fibrosis. J Clin Microbiol 1996; 34: 584-587. [PubMed]
Littlewood JM, Smye SW, Cunliffe H. Aerosol antibiotics in cystic fibrosis. Arch Dis Child 1993; 68: 788-792. [PubMed]
Peckham D, Williams K, Wynne S, Denton M, Pollard K, Barton R. Fungal contamination of nebuliser devices used by people with cystic fibrosis. [PubMed]
Smye SW, Shaw A, Norwood AH, et al. Some factors influencing the efficiency of a jet nebuliser system. Clin Phys Physiol Meas 1990; 11: 167-175. [PubMed]
Walsh NM, Casano AA, Manangan LP, et al. Risk factors for Burkholderia cepacia complex colonisation and infection among patients with cystic fibrosis. J Pediatr 2002; 141: 512-517. [PubMed]
