
2007
Ian M Balfour-Lynn. Cystic fibrosis papers of the year 2007. J R Soc Med 2008 Jul;101 Suppl 1(Suppl 1):S10-4.doi: 10.1258/jrsm.2008.s18004. Free PMC article pubmed.ncbi.nlm.nih.gov/18607013/

Ian Balfour-Lynn
The literature search was conducted using PubMed (www.ncbi.nlm.nih.gov/sites/entrez), entering the search term ‘cystic fibrosis’, for the period 1 January to 13 November 2007. There were 1228 papers identified; when limits were set for ‘clinical trial’ and ‘randomized controlled trial’ 63 papers remained. In addition, three meta-analyses were identified. Ian Balfour-Lynn indicates the papers selected for this review are a personal choice of studies with important or interesting clinical messages.
The full text, via pubmed.ncbi.nlm.nih.gov/18607013/ is recommended
2007
Barth AL, de Abreu E Silva FA, Hoffmann A, Vieira MI, Zavascki AP, Ferreira AG, da Cunha LG Jr, Albano RM, de Andrade Marques E. Cystic fibrosis patient with Burkholderia pseudomallei infection acquired in Brazil. Microbiology 2007; 45:4077-80. [PubMed]

Fig. 1 Afonso Luis Barth. faberge
Burkholderia pseudomallei is rarely isolated from patients with CF outside known areas of endemicity. These authors report the recovery of B. pseudomallei from the sputum of a cystic fibrosis patient who lives in Brazil and highlight the importance of careful attention to unusual non-fermentative gram-negative rods in cystic fibrosis patients (also Visca et al. 2001 above for Thailand experience).
Dr Afonso Luis Barth is at Unidade de Microbiologia e Biologia Molecular, Serviço de Patologia Clínica, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil.
Basu AP. Kumar P. Devlin AM. O’Brien CJ. Cystic fibrosis presenting with bilateral facial palsy. Eur J Paediatr Neurol 2007; 11:240-242. [PubMed]

Fig. 2 Anna Basu @AnnaBasu
A 15-week old male infant presented with bilateral lower motor neuron facial palsy of unknown cause. Subsequently his growth deteriorated and he developed progressively worsening cough and wheeze. A diagnosis of cystic fibrosis was confirmed and hypovitaminosis A detected. Improvement of the facial palsy was noted following standard management of cystic fibrosis including vitamin A supplementation.
– Presentations due to the manifestations of vitamin deficiency, particularly of vitamin A, are well documented. A similar case in a 10 week old infant was reported with low vitamin A levels and bilateral facial nerve paralysis (Cameron C et al. J Cyst Fibros 2007; 6:241-243. [PubMed]).
Dr Anna P Basu (fig. 2) is a senior lecturer and honorary consultant paediatric neurologist at the Royal Victoria Infirmary, Queen Victoria Road, Newcastle upon Tyne, UK.
Battezzati A, Battezzati PM, Costantini D, Seia M, Zazzeron L, Russo MC, Dacco V, Bertoli S, Crosignani A, Colombo C. Spontaneous hypoglycaemia in patients with cystic fibrosis. Euro J Endocrinol 2007; 156:369-376. [PubMed]

Fig.3 Alberto Battezzati Auxologica
A total of 129 CF patients without stable diabetes received 188 oral glucose tolerance tests. Fasting plasma glucose of < 60 mg/dl (3.3 mmo/l) was detected in 14% of studies and reactive hypoglycaemia (PG < 50 mg/dl (2.8 mmo/l)) in 15%. The oral glucose tolerance test-based diabetes frequency was similar in the lowest quartile (Q1) and Q2-3 for fasting plasma glucose (10 and 8%), with higher glucose increment and area under the curve in Q1. Insulin and C-peptide levels were similar among fasting plasma glucose (FPG) quartiles. Those with class I CFTR mutations had higher insulin concentrations than class II, especially in Q1 for FPG. Lower FPG was associated with more frequent hospitalization rates and lower Shwachman scores.
– Fasting asymptomatic hypoglycaemia is frequent and possibly related to inappropriate insulin secretion control in class I mutation carriers. Low fasting plasma glucose does not exclude impaired glucose tolerance and diabetes in CF and does reflect worse clinical status.
Prof. Alberto Battezzati (fig.3) is at the Department of Pediatrics, CF Center, Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, Italy.
Brochet MS, McDuff AC, Bussières JF, Caron E, Fortin G, Lebel D, Marcotte JE. Comparative efficacy of two doses of nebulized colistimethate in the eradication of Pseudomonas aeruginosa in children with cystic fibrosis. Canad Resp J 2007; 14:473-479. [PubMed]

Fig 4. Marie-Sophie Brochet
ResearchGate
A study to compare the efficacy of two doses of nebulized colistimethate (30 mg versus 75 mg twice daily) for the eradication of P. aeruginosa in children with CF and intermittent colonization. There was no statistically significant difference in the rate of eradication of P. aeruginosa at days 28 and 90, neither when comparing the doses of colistimethate nor duration of treatment.
– Most antibiotic regimens for eradicating early P. aeruginosa infection seem to achieve about an 80% success. The time to reinfection varies and will depend on the environmental exposure of the individual to P. aeruginosa as it has been shown that most “recurrences” are usually new infections with a different strain of the organism rather than recurrences with the same organism.
Marie-Sophie Brochet (fig.4) is in the Dept. of Pharmacy, CHU Sainte-Justine, Montreal, Canada
Boucher RC. Evidence for airway surface dehydration as the initiating event in airway disease. J Int Med 2007; 261:5-16.[PubMed]

Fig. 5 Richard Boucher
A very clear review marshalling the relevant current experimental and clinical evidence for Richard Boucher’s too little salt theory. Airway surface dehydration due to excessive sodium absorption and reduced chloride secretion is the main cause of the CF lung being unable to defend itself against infection. The hypothesis predicts the airway surface dehydration produces the mucus adhesions, inflammation and bacterial biofilm formation characteristic of cystic fibrosis.
Professor Richard Boucher (fig.5) is Professor and Director Cystic Fibrosis/Pulmonary Research Centre at the University of North Carolina, Chapel Hill. He has for many years been a leader in CF research and long time protagonist of the “low salt theory”. The theory is convincing.
– The development of lung disease in transgenic ENaC-over expressing mouse models has been impressive suggesting that the early spectrum of CF-like lung disease is related to dehydration in the airways. Inhaled hypertonic saline, drawing water into the airways, has shown significant clinical benefit in people with CF.
Bertenshaw C, Watson AR, Lewis S, Smyth A. A survey of acute renal failure in cystic fibrosis patients in the United Kingdom. Thorax 2007; 62:541-545. [PubMed]
Between 1997 and 2004, 26 of the 55 identified cases of acute renal failure in the UK consented to data extraction and 24 had acute renal failure (ARF). In 21 cases (88%) an aminoglycoside, usually gentamicin was prescribed at the onset of ARF or in the preceding week. This study implicates intravenous aminoglycosides, particularly gentamicin, in the aetiology of acute renal failure in cystic fibrosis.
– This is an important study of one of the long term complications of life-long therapy with potentially toxic drugs such as aminoglycosides with particular emphasis on the use of gentamicin.
The use of routine intravenous gentamicin for people with CF represents suboptimal therapy as it is less effective against Pseudomonas, more ototoxic and more nephrotoxic than tobramycin. Many of the treatments, used repeatedly for many years, have renal side effects including cyclosporine, the immunosuppressant used after lung transplantation.
The CF Trust Antibiotic Working Group advise that intravenous gentamicin should no longer be used in people with cystic fibrosis.
Dr Carol Bertenshaw is in the Academic Division of Respiratory Medicine, University of Nottingham, Clinical Sciences Building, and Department of Paediatrics, Nottingham City Hospital, Hucknall Road, Nottingham
Boesch RP, Acton JD. Outcomes of fundoplication in children with cystic fibrosis. J Pediatr Surg 2007; 42:1341-1344. [PubMed]

Fig.6 Richard P Boesch.
Twenty five children with CF underwent fundoplication to treat gastro-oesophageal reflux. 12% had complications that required a subsequent surgical procedure. Although 28% were able to discontinue their anti-reflux medications, no less than 48% developed symptoms of recurrent gastro-oesophageal reflux. Only children who had an FEV1 of less than 60% predicted at the time of fundoplication exhibited an improvement in FEV1 slope.
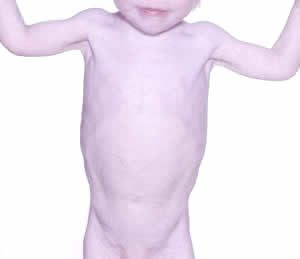
Fig 7. Wasting due to chronic reflux cured by fundoplication. Author’s photo
The complication rate of fundoplication is similar to that reported in large series in children without CF. There is a high rate of recurrent reflux and little apparent benefit for either nutritional or pulmonary outcomes. However, an individual child may benefit greatly from the procedure e.g. fig. 7 – this child had severe reflux causing uncontrollable vomiting, severe oesophagitis and progressive weight loss. He had a fundoplication after which his symptoms and general progress improved.
Dr Richard Paul Bosch (fig.6) is in the Division of Pediatric Pulmonary Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA
Bruzzese E, Raia V, Spagnuolo MI, Volpicelli M, De Marco G. Maiuri L, Guarino A. Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis. Clin Nutr 2007; 26:322-328.pubmed.ncbi.nlm.nih.gov/17360077/
Nineteen children with CF received the probiotic Lactobacillus GG (LGG) for 6 months and then changed to a placebo of oral rehydration solution (ORS) for 6 months; in parallel nineteen received ORS and then changed to LGG. Patients treated with LGG showed a reduction of pulmonary exacerbations (Median 1 vs. 2) and of hospital admissions (Median 0 vs. 1, range 3 vs. 2,). LGG resulted in a greater increase in FEV1 (3.6% +/- 5.2 vs. 0.9% +/- 5; p=0.02) and body weight (1.5 kg +/- 1.8 vs. 0.7 kg +/- 1.8; p=0.02). The authors concluded that Lactobacillus GG reduces pulmonary exacerbations and hospital admissions in patients with CF, that probiotics may delay respiratory impairment and that a relationship exists between intestinal and pulmonary inflammation.
– There is an increasing interest on the part of patients, parents and doctors in the role probiotics in treating people with CF (Borowitz D et al, J Pediatr Gastroenterol Nutr 2005; 41:273-285. [PubMed]); however, as yet, few CF clinics advise their routine use, perhaps because the evidence of their value is still sparse; but also there are so many other components to treatment that there is reluctance to add yet another medicine to gain a marginal benefit.
— There continued to be a steady output on the use of probiotics (115 publications by 2023) but the evidence is not considered adequate to recommend their routine use.[pubmed.ncbi.nlm.nih.gov/35956335/]
Dr Eugenia Bruzzese is in the Department of Pediatrics, University of Naples “Federico II”, Via S. Pansini 5, 80131 Naples, Italy.
Broughton-Head VJ, Shur J, Carroll MP, Smith JR, Shute JK. Unfractionated heparin reduces the elasticity of sputum from patients with cystic fibrosis. Am J Physiol – Lung C 2007; 293:L1240-9. [PubMed]

Fig 8. Janice Shute
Unfractionated heparin (UFH) significantly reduced the elasticity and yield stress, but not the viscosity, of CF sputum from patients not receiving dornase alfa therapy. Heparin dose-dependently significantly increased the diffusion of nanospheres through CF sputum from patients not on dornase alfa therapy from 10.5 +/- 2.5% at baseline to 36.9 +/- 4.4% at 10 mg/ml but was more potent, with maximal effect at 0.1 mg/ml, in patients who were on dornase alfa therapy. Thus the mucoactive properties of UFH indicate its potential as a new therapeutic approach in patients with cystic fibrosis.
– There were subsequent studies from Southampton and Portsmouth in the UK although an earlier pilot study using inhaled heparin 50,000 IU twice daily showed no clinical effect – a larger dose was suggested.[PubMed]. Also cospray-dried unfractionated heparin dry powder was suggested as a more efficient preparation to develop as a mucolytic for people with CF (Shur J et al. J Pharm Sci 2008; 97:4857-4868. [PubMed]).
Dr Janis Shute (fig. 8) is Professor in Pharmacology, Pharmacy and Biomedical Sciences in Portsmouth UK and has been involved with CF research in collaboration with the CF centre in Southampton as in this present study, also as advisor to the CF Trust on scientific matters in the Research and Medical Advisory Committee.
Childers M, Eckel G, Himmel A, Caldwell J. A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates. Medical Hypotheses 2007; 68:101-112. [PubMed]

Fig 9. Melanie Childers
American Wellness
Understanding that the cystic fibrosis transmembrane conductance regulator (CFTR) is responsible for glutathione (GSH) transport, the authors hypothesize that mutations of the CFTR, which create abnormal GSH transport, will lead to aberrations of GSH levels in both the intracellular as well as the extracellular milieu. These alterations in normal cellular GSH levels affect the redox state of the cell, thereby affecting the intracellular stress protein, metallothionein. The authors describe how this disruption of the redox state caused by excess cellular GSH, will naturally prevent the delivery of zinc as a cofactor for various enzymatic processes, and how these disruptions in normal redox may cause alterations in both humoral and cell-mediated immunity. Moreover, the symptom of thick sticky mucus in these patients might be explained through the understanding that oversulfation of mucus is a direct result of elevated cellular GSH and cysteine. The issues of hyperinflammation, altered pH and the imbalance of fatty acids that are typical in cystic fibrosis are addressed-all of which may also be linked to disruptions in GSH homeostasis. Additionally, this new model of cystic fibrosis pathology, clarifies the relationship between the CFTR and the multi-drug resistance proteins, and the lack of cell-mediated immunity by predicting that the substrate of these proteins is a glutathione adduct of thiocyanate. Finally, a new therapeutic strategy by using isothiocyanates to rectify the GSH imbalance and restore the immune system is suggested for the treatment of cystic fibrosis patients.
– Further work proposing a role for glutathione.
Dr Melanie Childers (fig.9) is a Consultant in Internal Medicine at Share International Foundation, 1720 205th Pl NE, Sammamish, WA 98074, USA
Chuchalin A, Csiszer E, Gyurkovics K, Bartnicka MT, Sands D, Kapranov N, Varoli G, Monici Preti PA, Mazurek H. A formulation of aerosolized tobramycin (Bramitob) in the treatment of patients with cystic fibrosis and Pseudomonas aeruginosa infection: a double-blind, placebo-controlled, multicenter study. Paediatr Drugs. 2007; 9 Suppl 1:21-31. [PubMed]

Fig. 10 Alexander ChuchalinThe Sun
The purpose of this trial was to assess the efficacy and tolerability of a relatively recently introduced preparation of tobramycin highly concentrated solution for inhalation (TSI) [300mg/4mL; Bramitob] when added to other anti-Pseudomonal therapies in CF patients with chronic P. aeruginosa infection. A total of 247 patients were randomized in the study. No significant changes in serum creatinine and auditory function were detected. Long-term, intermittent administration of this aerosolized tobramycin formulation (300mg/4mL) in CF patients with chronic P. aeruginosa infection significantly improved pulmonary function and microbiologic outcome, decreased hospitalizations, increased nutritional status, and was well tolerated.
– This preparation appears to be the same or very similar to TOBI and therefore it is not surprising that the results are similar (Poli G, et al, 2007. [PubMed]; Lenoir G et al. 2007. [PubMed]); hopefully the cost will be less.
Prof. Alexander Chuchalin (fig 10) is at the Scientific Research Pulmonology Institute, Russian State Medical University, Moscow, Russia. In 2020 Chuchalin, described as Russia’s top respiratory doctor, resigned after criticising release of the new untested SputnikV coronavirus vaccine.
Clancy JP, Rowe SM, Bebok Z, Aitken ML, Gibson R, Zeitlin P, Berclaz P, Moss R, Knowles MR, Oster RA, Mayer-Hamblett N, Ramsey B. No detectable improvements in cystic fibrosis transmembrane conductance regulator by nasal aminoglycosides in patients with cystic fibrosis with stop mutations. Am J Resp Cell Mol 2007; 37:57-66. [PubMed]

Fig 11. John Clancy
A US multicenter study was conducted in two cohorts of patients with CF, those heterozygous for stop mutations in the CFTR gene and those without nonsense mutations, to investigate the effects of both gentamicin and tobramycin administered over a 28-day period on sequential nasal potential difference and airway cell immunofluorescence endpoints.
Eleven patients with CF who had stop mutations were enrolled in a randomized, double-blinded, crossover fashion to receive each drug, while 18 subjects with CF without stop mutations were randomized 1:1 in a parallel fashion to receive one drug. After demonstration of drug delivery, neither aminoglycoside produced detectable changes in nasal ion transport or CFTR localization in brushed cells from either study group.
These results with first-generation suppressive agents suggest the need for improved drug delivery methods and/or more potent suppressors of nonsense mutations to confer CFTR correction in subjects with CF heterozygous for nonsense mutations. The study provides valuable information on parameters of the nasal potential difference measurements for use in future multicenter clinical trials.
The results appear to conflict with earlier work of Wilschanski et al (2000 and 2003 above) but variability of response has been attributed to the efficiency of nonsense-mediated mRNA decay which may vary and hence have an important role in governing the response to treatments aiming to suppress nonsense mutations. (2007 Linde L, et al. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J Clin Invest 2007; 117:683-692. [PubMed]).
Professor John Clancy (fig. 11) is a leader in CF research and has played a major role in many CF research groups and committees. He is Research Director, Division of Pulmonary Medicine and Professor of the Department of Pediatrics, Cincinnati Children’s.
Corno V, Dezza MC, Lucianetti A, Codazzi D, Carrara B, Pinelli D, Parigi PC, Guizzetti M, Strazzabosco M, Melzi ML, Gaffuri G, Sonzogni V, Rossi A, Fagiuoli S, Colledan M. Combined double lung-liver transplantation for cystic fibrosis without cardio-pulmonary by-pass. Am J Transpl2007; 7:2433-2438. [PubMed]
The authors performed sequential bilateral single lung-liver transplantation – a therapeutic option for patients with end stage lung and liver disease. They performed the operation in three young men affected by CF. All the recipients had respiratory failure and portal hypertension with hypersplenism. The three recipients are alive with a median follow-up of 670 days (range 244-1,533). The operation is a complex but effective procedure for the treatment of end stage lung disease due to CF.
Dr V Corno is at General Surgery III Liver and Lung Transplantation Center, Ospedali Riuniti, Bergamo, Italy.
Coulthard KP, Peckham DG, Conway SP, Smith CA, Bell J, Turnidge J. Therapeutic drug monitoring of once daily tobramycin in cystic fibrosis – caution with trough concentrations. J Cyst Fibros 2007; 6:125-130. [PubMed]

Fig 12. Peter Bye and Kingsley Coultard Author’s photo
Once daily intravenous aminoglycoside dosing (ODD) is widely used to treat acute Pseudomonas aeruginosa exacerbations in patients with cystic fibrosis. Controversy exists as to what is the most appropriate method of therapeutic drug monitoring (TDM) of such therapy with recommendations including trough plasma concentrations of <1 mg/L or <2 mg/L, area under curve (AUC) and various nomograms.
After the study the authors concluded that “Area under the curve (AUC) as the method of therapeutic drug monitoring may not only reduce toxicity but also optimise efficiency”.
Kingsley Coultard (fig. 12) is a widely-travelled pharmacologist from Adelaide who has visited many CF centres including Leeds on a number of occasions.
De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 2007; 6:75-78. [PubMed]

Fig. 13 Frans de Baets UZ Gent
One of the many relatively new pathogens encountered in people with CF. Although there was more need for intravenous antibiotic treatment courses, no faster decline in lung function was observed in A. xylosoxidans positive patients. (Hansen CR, et al. 2006 above from Copenhagen – there were similar findings but more treatment was required particularly in those patients with rising antibodies).
Prof. Frans De Baets (fig.13) is at the Cystic Fibrosis Centre, University Hospital Ghent, De Pintelaan 185, 9000 Ghent, Belgium.
Deterding RR, Lavange LM, Engels JM, Mathews DW, Coquillette SJ, Brody AS, Millard SP, Ramsey BW, for the Cystic Fibrosis Therapeutics Development Network and the Inspire 08-103 Working Group. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am J Resp Crit Care 2007; 176:362-369. [PubMed]

Fig. 14 Robin Deterding. ATS news-America
Denufosol tetrasodium is a selective P2Y(2) agonist that enhances mucosal hydration and mucus clearance by activating Cl(-) secretion and inhibiting epithelial Na(+) transport through a non-cystic fibrosis transmembrane conductance regulator mechanism in the lung. The study was a randomized, double-blind, multi-center, 28-day, phase 2 clinical trial of denufosol tetrasodium inhalation solution (20, 40, or 60 mg) versus placebo (normal saline). Denufosol patients (pooling active doses) had significantly higher changes from baseline in respiratory function than placebo patients at the end of the study. The authors concluded that Denufosol administered three times daily for 28 days appeared to be safe and well tolerated in this population with mild cystic fibrosis and provided preliminary evidence of potential benefit in lung function.
This is one of a number of drugs showing initial promise to correct or reduce the effects of the basic defect on the airway surface liquid (also Deterding et al, 2005 above). In June 2008, Inspire announced the results of its first Phase 3 trial with Denufosol (Tiger 1). The trial demonstrated statistical significance for its primary endpoint of change in FEV1 from baseline compared to placebo. By late 2010 Inspire had completed enrolment to a further Phase 111 trial (Tiger 2).
Unfortunately this last trial showed that treatment with denufosol for 48 weeks did not improve pulmonary function or reduce the incidence of pulmonary exacerbations (Ratjen F et al. J Cyst Fibros 2012;11 :539-549[PubMed]) and further development of the drug was halted.
Robin Deterding (fig. 14) is Chair, of Children’s Interstitial Lung Disease Research Network and Chief, Pediatric Pulmonary Medicine Children’s Hospital Colorado.
De Boeck K, De Baets F, Malfroot A, Desager K, Mouchet F, Proesmans M. Do inhaled corticosteroids impair long-term growth in prepubertal cystic fibrosis patients? Eur J Pediatr 2007:166:23-28. [PubMed]

Fig. 15. Kris De Boeck
The effect of inhaled corticosteroids (ICS) on lung function and other clinical variables was studied in 27 prepubertal CF children with mild to moderate lung disease. In a prospective double-blind case-controlled study, fluticasone propionate 500 microg or placebo were administered twice daily during 12 months. There was no statistically significant difference in the evolution of lung function and the number of respiratory exacerbations between groups.
However, longitudinal growth in fluticasone patients was significantly slower than in placebo patients: 3.96 (0.29) cm versus 5.49 (0.38) cm [p<0.005, analysis of variance (ANOVA)] over the 12-month study duration. This resulted in a significant change in height standard deviation score (SDS) of -0.38 (0.09) in the fluticasone group versus -0.01 (0.07) in the placebo group (p<0.003, ANOVA). No catch-up growth was noted 1-2 years after discontinuation of inhaled steroids. The authors concluded the use of high-dose ICS in CF patients with mild lung disease may lead to persistent growth impairment.
It is no surprise that this dose of fluticasone leads to impairment of growth – apparently in this study without any obvious clinical benefit. Although we described clearly impaired growth over 20 years ago in some asthmatic children taking inhaled steroids (Littlewood et al, 1988. Growth retardation in asthmatic children treated with inhaled beclomethasone diproprionate. Lancet 1988;865. [PubMed]) there has been a reluctance to accept our findings by many respiratory paediatricians. However, clinicians who carefully follow children who are taking substantial doses of inhaled steroids for prolonged periods and who measure and chart their growth accurately observe the quite obvious slowing of growth in some children. In fact, we have observed virtual arrest of height growth in young children with CF treated with “heroic doses” of fluticasone to the extent that invasive dietary intervention was suggested! Of course, in such children the weight SD or centile for weight is usually much better than that for height.
Prof. Kris De Boeck( fig.15) is Head of Paediatric Pulmonology and infectious Diseases and Head of CF Reference Center, Leuven
Desmond CP, Wilson J, Bailey M, Clark D, Roberts SK. The benign course of liver disease in adults with cystic fibrosis and the effect of ursodeoxycholic acid. Liver Int 2007; 27:1402-1408. [PubMed]

Fig. 16 Christopher Paul Desmond
healthpages.wiki
The authors concluded that liver disease in 6.7% of 278 adults with CF is commonly complicated by portal hypertension (67%), but morbidity and mortality associated with this in their patient population were low. Ursodeoxycholic acid (URSO) treatment was associated with improvement in hepatobiliary symptoms and liver function tests (50%).
This is a record of experience of CF related liver disease at a large adult CF unit; similar conclusions also came from experience at the adult CF centre in Cambridge UK (Nash et al, 2008 above). Although the Cochrane review found insufficient evidence for the use of ursodeoxycholic acid, most CF Centres treat people with CF who have evidence of liver disease with URSO.
Christopher Paul Desmond (fig. 16) was at the Department of Gastroenterology, The Alfred Hospital, Melbourne, Vic., Australia.
Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947-2003. Eur Respir J 2007; 29:522-526. [PubMed]
Data up to 1995 on the survival of 3-year cohorts of patients with CF born in the UK in the period 1968-1992 had previously been published (Dodge et al. Arch Dis Child 1997; 77:493-496.[PubMed]).

Fig. 17 John Dodge
Linkedin
The present study, coordinated by John Dodge (fig. 17) is the last from the original Survey of the UK CF Working party, reports survival data up to the end of 2003 together with a 2003 population estimate. All subjects with CF born in the UK in the period 1968-1992 were identified up to 1997 and information from the death certification authorities up to the end of 2003 was added.
The observed survival up to 2003 of CF patients born in 1978 was 55% for males and 49% for females. For 1988 and 1992 the data were 91 and 88%, and 97 and 96%, respectively. The estimated 2003 mid-year CF population was 8,284.
The continuing improvement in survival of cystic fibrosis patients in successive cohorts suggests the previous prediction of median survival of more than 50 years of age for individuals born in 2000 continues to look realistic, even in the absence of proven effective therapy aimed at correcting the basic cystic fibrosis defect.
Döring G, Meisner C, Stern M, for the Flagella Vaccine Trial Study Group. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proceedings of the National Academy of Sciences of the United States of America 2007; 104:11020-5. [PubMed]

Fig. 18 Gerd Döring
In a double-blind, placebo-controlled, multicenter trial, 483 European patients, 2-18 years of age without P. aeruginosa colonization were randomly assigned to receive four intramuscular injections of a bivalent P. aeruginosa flagella vaccine or placebo over a 14-month period. Patients were evaluated quarterly for P. aeruginosa-positive throat cultures and antipseudomonal serum antibody titers during the study period of 2 years. The vaccine was well tolerated, and the patients developed high and long-lasting serum antiflagella IgG titers. In the intention-to-treat group (all patients enrolled), 82 of 239 vaccinated patients had P. aeruginosa infection and/or antipseudomonal serum titers compared with 105 of 244 patients in the placebo group (P = 0.05; relative risk: 0.80; 95% CI: 0.64-1.00). Analysis of the 381 patients in the per-protocol group, who received all four vaccinations or placebo treatments, revealed 37 of 189 patients with infection episodes in the vaccine group compared with 59 of 192 patients with such episodes in the placebo group (P = 0.02; relative risk: 0.66; 95% CI: 0.46-0.93). P. aeruginosa strains, exhibiting flagella subtypes included in the vaccine, were significantly less frequently isolated from vaccinates than from placebo controls (P = 0.016, relative risk: 0.319; 95% CI: 0.12-0.86). Chronic P. aeruginosa infection was rare because of recent institution of early antibiotic eradication regimes. Active immunization of patients with cystic fibrosis lowers the risk for infection with P. aeruginosa and therefore may contribute to a longer survival of these patients.
– As in a similar trial of vaccine to protect against P. aeruginosa (Aerugen) the chance of showing a convincing result was reduced in part by the increasingly widespread use of early eradication regimens resulting in a falling prevalence of chronic Pseudomonas infection. I’m not aware of any people with CF receiving Pseudomonas vaccines at present (2023).
Etherington C, Bosomworth M, Clifton I, Peckham DG, Conway SP. Measurement of urinary N-acetyl-b-D-glucosaminidase in adult patients with cystic fibrosis: before, during and after treatment with intravenous antibiotics. J Cyst Fibros 2007; 6;67-73.[PubMed]

Fig. 19 Christine Etherington. author’s photo
Both tobramycin and colistin cause mild acute renal tubular injury with a significant rise in urinary NAG excretion. Patients with CFRD seem to be at greatest risk of renal tubular damage. Cumulative damage is evident with repeated dosing. Previous exposure to nephrotoxic antibiotics, especially colistin, is associated with elevated baseline NAG levels. The authors recommend that intravenous colistin is reserved for patients with resistant Pseudomonas aeruginosa or those who are intolerant to tobramycin. Serial longitudinal NAG measurements may be useful in patients with CF, especially those with CFRD, to identify patients at risk of developing renal disease.
Measurement of urinary NAG in relation to aminoglycoside treatment has been periodically studied since our first European conference report in 1984 (Miller MG et al. Nephrotoxicity of aminoglycosides. In: Lawson D ed. Cystic fibrosis: horizons. John Wiley & Sons Chichester 1984; 271; also Glass S et al. J Cyst Fibros 2005; 4:221-225. [PubMed]; Ring E et al. Arch Dis Child 1998; 78:540-543. [PubMed]). All these previous studies have shown transient rises of urinary NAG during aminoglycoside treatment indicating some tubular injury which recovers after the treatment; although in the Miller et al study the rise in NAG was increasingly greater with each additional course of aminoglycosides.
With increasing longevity of people with CF the cumulative effect of these repeated minor renal injuries are likely to become more relevant as evidenced by the increasing problem of renal failure in people with CF (Smyth A et al. Case-control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax 2008; 63:532-535.18245146 [PubMed]). In some, the effect of repeated courses of intravenous aminoglycosides is worsened by the immunosuppressive drugs required after lung transplantation.
Interestingly, urinary NAG levels were observed to rise after prolonged nebulised gentamicin used (successfully it must be added) to prevent new Pseudomonas infection in children with CF and considered to present a risk of renal toxicity (Ring et al. Arch Dis Child 1998; 78:540-543. [PubMed]). However, subsequent publications on the long term use of nebulised gentamicin in non-CF bronchiectasis consider there to be negligible absorption and the treatment suitable for children (Twiss TJ et al, 2005. [PubMed]) and adults (Murray M P, et al, 2010. [PubMed]). In the UK it is advised that both nebulised and intravenous gentamicin are avoided in people with CF (Antibiotic Treatment for Cystic Fibrosis. CF Trust. 3rd edition, 2009).
Dr Christine Etherington (Fig 19) is a senior physician in the Leeds Regional Adult CF Centre. Christine has made a major contribution to research and patient care at the Leeds Adult CF Centre over many years.
Fennell PB, Quante J, Wilson K, Boyle M, Strunk R, Ferkol T. Use of high-dose ibuprofen in a pediatric cystic fibrosis center. J Cyst Fibros 2007; 6:153-158. pubmed.ncbi.nlm.nih.gov/16844429/
Despite its reported benefits, high-dose ibuprofen has been infrequently prescribed for children with cystic fibrosis. Nearly half of the patients in this pediatric cystic fibrosis center who were prescribed with high-dose ibuprofen discontinued therapy due to adverse events, not because of poor adherence or patient choice. Neither use of high-dose ibuprofen nor its cessation resulted in a significant change in the rate of decline in pulmonary function or influenced hospitalization rates.
– This is further confirmation that ibuprofen has failed to make a significant contribution to CF care (also Konstan et al, 1995 above; Lands et al, 2007 above supporting the use of ibuprofen). The benefits were modest, blood levels were required and the side effects relatively frequent.
Dr. Preston Blain Fennell is at the Mallinckrodt Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri, United States.
Figueredo J, Limberis MP, Wilson JM. Prediction of cellular immune responses against CFTR in patients with cystic fibrosis after gene therapy. Am J Resp Cell Mol 2007; 36:529-533. [PubMed]
Efficient delivery and transgene expression are not the only parameters that may influence the success of gene therapy. Host-specific immune responses generated against the therapeutic CFTR protein may pose a problem, especially when the coding sequence between the normal CFTR and mutated CFTR differ. This phenomenon is more pertinent to class I mutations in which large fragments of the protein are not expressed. However, T cells directed against epitopes that span sequences containing class II-V mutations are also possible. The authors used MHC-binding prediction programs to predict the probability of cellular immune responses that may be generated against CFTR in DeltaF508 homozygote patients. Results obtained from running the prediction algorithms yielded a few high-scoring MHC-Class I binders within the specific sequences, suggesting that there is a possibility of the host to mount a cellular immune response against CFTR, even when the difference between therapeutic and host CFTR is a single amino acid (F) at position 508.
This is an interesting paper as transient inflammatory reactions appear to be a frequent occurrence and a problem following the administration of gene therapy to people with CF.
Juanita Figueredo is with the Gene Therapy Program, Department of Pathology and Laboratory Medicine, Division of Transfusion Medicine, University of Pennsylvania, Philadelphia, PA 19104-3403, USA.
Fischer S, Bohn D, Rycus P, Pierre AF, de Perrot M, Waddell TK, Keshavjee S. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO) registry. J Heart & Lung Transplantation 2007; 26:472-477. [PubMed]
Some patients with severe primary graft dysfunction (PGD) after lung transplantation (LTx) require gas exchange support using an extracorporeal membrane oxygenator (ECMO) as a life-saving therapy.The ELSO registry currently includes 31,340 ECMO cases, of which 151 were post-LTx patients with primary graft dysfunction (PGD) 15 had cystic fibrosis. ECMO was discontinued in 93 patients owing to lung recovery. In total, 63 (42%) patients survived the hospital stay.Although the ELSO registry was not primarily established to study ECMO in LTx, it provides valuable insights and evidence that there is indeed an appreciable in some patients for the use of ECMO for primary graft dysfunction after lung transplantation.Clearly, this is a very high-risk patient population, and no single centre can accumulate a large experience of ECMO for primary graft dysfunction after lung transplantation.
Stefan Fischer is with the Toronto Lung Transplant Program, Division of Thoracic Surgery, Toronto General Hospital, University Health Network, Toronto, Ontario, Canada.
Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Amer J Respir Crit Care Med 2007; 176: 957-969. [PubMed]

Fig 20. Patrick Flume
In 2005 the Cystic Fibrosis Foundation established a committee to examine the clinical evidence for each therapy used for CF and to provide guidance for the prescription of these therapies. The committee members developed and refined a series of questions related to drug therapies used in the maintenance of pulmonary function. Questions were addressed in one of three ways, based on available evidence: (1) commissioned systematic review, (2) modified systematic review, or (3) summary of existing Cochrane review
The strength of the evidence and the estimate of benefit were recorded and the recommendations graded. Grade A-good evidence and substantial benefit. Grade B- fair evidence, benefit outweighs harm. Grade C – no recommendation, benefits and harm balance too close. Grade D – fair evidence not effective or harm outweighs benefit. Grade I – insufficient evidence”.
The treatments received the following grades- Nebulised tobramycin – grade A for severe, grade B for mild persistent Pseudomonas infection; evidence for other inhaled antibiotics insufficient; (surprisingly even in 2005 the important area of early Pseudomonas treatment was not dealt with); rhDNase grade A recommendation; hypertonic saline grade B; inhaled and oral steroids both grade D;
non-steroidal anti-inflammatory drugs grade B; leukotriene modifiers insufficient evidence ; macrolides grade B;
prophylactic anti-staphylococcal antibiotics grade D; ß-agonist bronchodilators grade B; anti-cholinergic bronchodilators Grade I; n-acetylcysteine grade I.
The treatments that have been the subject of a CF Foundation Phase III trial are awarded either A or B recommendation. It is quite surprising that even in 2007 the early eradication treatment of Pseudomonas is not considered in a document on inhaled therapy. Not all would agree with the grading of prophylactic anti-Staphylococcal antibiotics for which there is a European trial (Weaver et al, 1995 above) and a wealth of European experience.
Dr Patrick Flume (fig. 20) works at the Medical University of South Carolina where he is Professor of Medicine and Pediatrics. He is also Director of the CF Centre there. He is co-chair of the Pulmonary Practice Guidelines Committee of the CF Foundation and very active in CF care and research.
Fridge JL, Conrad C, Gerson L, Castillo RO, Cox K. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J Pediatr Gastroenterol Nutr 2007; 44:212-218.[PubMed]

Fig. 21 Jacqueline Fridge WebMD
Fifty patients, 25 pancreatic-insufficient CF study patients (mean age, 17 y) and 25 gastrointestinal clinic control patients (mean age, 15 y), completed a glucose-hydrogen breath test after an overnight fast. A positive breath test was defined as a fasting hydrogen > or =15 ppm or a rise of > or =10 ppm hydrogen over baseline during the test.
The prevalence of positive breath tests was higher in the CF study group (56%) than in the control group (20%) (P = 0.02). The mean fasting hydrogen levels of patients in the study and control groups were 22 and 5 ppm (P = 0.0001). The mean Quality of Life questionnaire scores were not significantly different between breath test-positive and -negative study patients. The use of azithromycin was associated with an increased risk of a positive breath test. Use of laxatives and inhaled ipratropium was associated with a decreased risk of a positive breath test.
– This study confirmed previous work that patients with CF were more likely to have elevated fasting hydrogen levels compared with controls. This suggests a high prevalence of small bowel bacterial overgrowth in CF patients. Also medications commonly used by CF patients may influence intestinal health.
Dr Jaqueline L Fridge (fig 21) is in the Division of Gastroenterology, Children’s Hospital and Research Center Oakland, Oakland,
Green A, Kirk J. Guidelines Development Group. Guidelines for the performance of the sweat test for the diagnosis of cystic fibrosis. Ann Clin Biochem 2007; 44(Pt 1):25-34.[PubMed]
A multidisciplinary group (representing various professional bodies and supported by the Cystic Fibrosis Trust) has developed evidence-based guidelines for the performance of the sweat test in the UK. The guidelines cover patient information, subject suitability, sweat collection, sweat analysis, quality, interpretation of results, and responsibility for testing and training. The guidelines were produced following a detailed literature search by the process described by the Royal College of Paediatrics and Child Health (RCPCH), using the Scottish Intercollegiate Guidelines Network 1 (SIGN 1) criteria to grade the evidence. Recommendations are graded A, B, or C, depending on the level of evidence. The grade B recommendations (there were no grade A recommendations) were subsequently appraised and endorsed as part of the RCPCH process, according to Appraisal of Guidelines for Research and Evaluation in Europe (AGREE). The recommendations are summarized in tabular form representing the final version incorporating the comments from the appraisal process.Both the appraisal comments and the full evidence base can be found on www.rcpch.ac.uk/publications/clinical_docs.html. The full guidelines can also be found on http://www.ukneqas.org.uk/guidelines.htm.

Fig 22. Sue Hill, Anne Green (centre) & Andrew Lansley
Professor Anne Green (fig.22) with Sue Hill, Chief Scientific Officer at DOH and Andrew Lansley Secretary of State for Health) was enrolled into the Healthcare Science Roll of Honour at the 2012 Healthcare Science Awards. Citation “Professor Anne Green was until her retirement, consultant in paediatric clinical biochemistry at the the University of Birmingham. It was she who instigated nationwide newborn screening in the UK for inherited metabolic diseases. Anne Green laid the foundation for practice that everyone today accepts as being an essential part of newborn screening”.
Garside JP, Kerrin DP, Brownlee KG, Gooi HC, Taylor JM, Conway SP. Low gammaglobulin subclass 2 levels in paediatric cystic fibrosis patients followed over a 2-year period. Pediatr Pulmonol 2007; 42:125-130. [PubMed]
The aim of this study was to relate serum immunoglobulin G2 subclass levels in a large paediatric population with cystic fibrosis, to clinical status and antibody levels to Haemophilus influenzae type b and Streptococcus pneumoniae and to observe any changes over a 2-year period. There was a reduction in the prevalence of low levels of IgG2 from 29% to 10% over the 2-year period. Low levels of IgG2 were not associated with any decline in clinical well-being. Low levels of IgG2 alone were associated with low antibody levels to S. pneumoniae. Low levels of IgG2 and low levels of antibody to H. influenzae and S. pneumoniae were not associated with any decline in clinical well-being. Children with high levels of IgG2 had worse lung function, worse Shwachman-Kulczcyki and Northern chest X-ray scores and higher levels of P. aeruginosa infection. Children with low IgG2 levels were not worse clinically compared to those with normal or high IgG2 levels. High IgG2 levels were associated with a worse clinical status.
Dr J P Garside is at the Department of Paediatrics, Huddersfield Royal Infirmary, the study is from the Leeds CF Centre.
Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol 2007; 42:307-313. [PubMed]

Fig. 23 David Geller
An evaluation of the pharmacokinetics and safety of tobramycin inhalation powder (TIP), a novel dry-powder formulation designed to deliver a high payload of tobramycin topically to the lungs for management of chronic Pseudomonas aeruginosa infections.The development of dry powder inhaled antibiotics represents an important advance in the treatment of chronic lung infections but is by no means a new idea (Krasno L et al. Inhalation of penicillin dust. Science 1947; 106:249-250. [PubMed] ;Goldman JM et al. Inhaled micronised gentamicin powder: a new delivery system. Thorax 1990; 45:939-940. [PubMed]; Watson A et al. Sputum gentamicin levels after delivery by Rotahaler and nebuliser in Escobar H et al.(eds.) Elsevier Science Publishers. 1993; 115-117)
David E Geller (Fig.23) is at the Nemours Children’s Clinic, Orlando, Florida 32806, USA
Gibson RL, Emerson J, Mayer-Hamblett N, Burns JL, McNamara S, Accurso FJ, Konstan MW, Chatfield BA, Retsch-Bogart G, Waltz DA, Acton J, Zeitlin P, Hiatt P, Moss R, Williams J, Ramsey BW. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr Pulmonol 2007; 42:610-623. [PubMed]

Fig.24 Ronald L Gibson Seattle Children’s
An open label, sequential cohort study of tobramycin for inhalation (TSI) in young children with CF to investigate duration of antimicrobial treatment effect. Culture based, lower airway Pseudomonas eradication was observed in the majority of subjects for up to 1-3 months following TSI treatment. The authors concluded that tobramycin solution for inhalation monotherapy was safe and could eradicate lower airway P. aeruginosa for up to 3 months after treatment in young children with CF.
— These results are not surprising and could have been predicted, as a number of European studies studies had already shown that inhaled tobramycin, even in a more modest dose of 80 mg twice daily, was effective in eradicating early P. aeruginosa (Weismann HG, et al. Pediatr Pulmonol 1998; 25:88-92. [PubMed]; Ratjen F, et al. Lancet 2001; 358:983-984. [PubMed]). The dose of inhaled tobramycin of 300mg twice daily seems formidable and perhaps unnecessarily large as judged by previous experience, particularly in view of the great cost (£10,000 per year in the UK if given on alternate months as recommended). Nevertheless this was an important study from a major CF centre in N. America which is likely to have accelerated the progress to early treatment of Pseudomonas there.
Dr Ronald Gibson (Fig.24) is at the Department of Pediatrics, University of Washington/Children’s Hospital & Regional Medical Center, Seattle,
Grasemann H, Stehling F, Brunar H, Widmann R, Laliberte TW, Molina L, Döring G, Ratjen F. Inhalation of Moli 1901 in patients with cystic fibrosis. Chest 2007; 131:1461-1466. [PubMed]

Fig. 25 Hartmut Grasemann. SickKids
Moli1901 (now called duramycin) stimulates an alternative Ca dependent chloride channel and may thus compensate for the CFTR deficiency in the airway epithelium of CF patients. A phase II, placebo-controlled, double-blinded, single-center, multiple (5 consecutive days), rising-dose (daily dose, 0.5, 1.5, or 2.5 mg of Moli1901) study in Germany was conducted to investigate the safety and tolerability of multiple doses of aerosolized inhaled Moli1901 in 24 patients with CF and stable lung disease. Moli1901 was well tolerated in all but one CF patient, in whom a transient significant decrease in FEV1 developed following inhalation, which resolved spontaneously, and in a second patient in whom transient throat numbness developed during drug inhalation. A significant improvement of FEV1 was observed in the group receiving treatment with 2.5 mg/d Moli1901 compared to the group receiving placebo. Moli1901 was not detected in the plasma of the highest dose group.
The authors concluded that the inhalation of Moli1901 up to a total cumulative dose of 12.5 mg appears to be safe in adult patients with CF. In addition, Moli1901 had a sustained beneficial effect on pulmonary function, which supports further studies of its efficacy in CF patients.
Further studies with the drug, now called duramycin, followed and suggested that duramycin was suitable for intrapulmonary administration in cystic fibrosis (Steiner I et al. Naunyn Schmiedebergs Arch Pharmacol 2008; 378:323-333. [PubMed]; Oliynyk I et al. APMIS 2010; 118:982-990. [PubMed]). In various publications the drug is referred to as Moli1901, duramycin and Lancovutide.
Dr Hartmut Grasemann (fig. 25) is at the The Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada.
Griese M, Latzin P, Kappler M, Weckerle K, Heinzlmaier T, Bernhardt T, Hartl D. alpha1-antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Resp J 2007; 29:240-250. [PubMed]

Fig. 26 Matthias Griese
This study showed that inhalation of alpha1-antitrypsin (AAT) for 4 weeks increased AAT levels and decreased the levels of elastase activity, neutrophils, pro-inflammatory cytokines and the numbers of P. aeruginosa in induced sputum. However, it had no effect on lung function. No difference was found between the peripheral and bronchial inhalation mode.
In conclusion, although no effect on lung function was observed, the clear reduction of airway inflammation after alpha (1)-antitrypsin treatment may precede pulmonary structural changes.
Although a previous trial of a natural product of alpha1-antitrypsin was encouraging (McElvaney et al, 1991 above), a more recent trial of the genetically engineered product was negative (Martin et al, 2006 above). However, this German trial provides further evidence of the expected beneficial effect of alpha 1-antitrypsin and may lead to further studies – although these were not evident by 2023.
Prof. Mathias Griese (Fig. 26) is with the Lung Research Group, Dr. von Hauner Children’s Hospital, Ludwig Maximilians University, Lindwurmstr 4, D-80337 Munich, Germany.
Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Resp Crit Care 2007; 175:816-821.[PubMed]

Fig. 27 Marcus Henke
Cystic fibrosis is believed to be associated with mucus hypersecretion; thus, the principal airway gel-forming mucins, MUC5AC and MUC5B, are also expected to be increased relative to non-CF secretions. However, these mucins are decreased during stable CF disease. In this study, the authors determine if these mucins increase during a pulmonary exacerbation of CF.
Expectorated sputum was collected from 11 adults with CF during stable disease and then during a pulmonary exacerbation and from 12 healthy control subjects. MUC5AC and MUC5B proteins were measured by Western blot. DNA content was measured using microfluorimetry.RESULTS: MUC5AC protein increased by 908% and MUC5B by 59% (p < 0.05 for both) during an exacerbation compared with periods of stable disease. During stable disease, the vol/vol quantity of MUC5AC protein was 89% less than normal mucus, and the mucin-associated sugars, measured using a lectin binding assay, were 46% less compared with normal mucus. The concentration of DNA in CF sputum did not increase during an exacerbation.
The authors concluded that during a CF exacerbation, concentration of secreted mucin increased to the amount found in mucus from normal subjects, suggesting that the capacity to secrete mucin in response to an infection or inflammatory stimulus is preserved in CF airways. This might help to protect the airway from injury. It is surprising that the DNA content of the sputum did not increase during exacerbations.
Marcus Henke (fig.27) is at Philipps-University Marburg, Department of Pulmonary Medicine, Baldingerstrasse 1, 35043 Marburg, Germany..
Hilliard TN, Sukhani S, Francis J, Madden N, Rosenthal M, Balfour-Lynn I, Bush A, Davies JC. Bronchoscopy following diagnosis with cystic fibrosis. Arch Dis Child 2007; 92:898-899.[PubMed]

Fig 28 Tom N Hilliard LinkedIn
The authors recently changed their practice and performed bronchoscopy following a diagnosis of cystic fibrosis. On a retrospective review of 25 children, Pseudomonas aeruginosa was detected in bronchoalveolar lavage for the first time in five children (20%) and Staphylococcus aureus in four (16%). Lavage culture was positive in eight of the 18 children without respiratory symptoms. The authors suggest that these findings highlight the potential of bronchoscopy following diagnosis, even in asymptomatic children.
Whether to recommend bronchoscopy in an asymptomatic screened infant with CF is dependent on many factors, not least, where the infant was born and the facilities and skill available for paediatric respiratory investigation. Also, the treatment policy of the unit responsible for the care of the infants. Also there is the potential danger of infecting an, as yet uninfected, infant with the instrument if sterilisation has been faulty; also hospitalisation itself does present a definite infection risk to infants with CF. If the infants were started on prophylactic flucloxacillin from diagnosis (as is recommended by the UK CF Trust’s expert Antibiotic Working Group 2009), it is very unlikely that S. aureus would have been cultured. Also units where screening has been routine for many years, such as Leeds, have managed to achieve very low levels of chronic Pseudomonas infection using only frequent throat cultures, cough swabs and serum antibody levels to recognise and treat early P. aeruginosa infection. For example, if an infant with CF had repeatedly negative upper respiratory tract cultures and negative Pseudomonas antibody levels it would be very unlikely there would be positive bronchial cultures.
So bronchoscopy for all at diagnosis, although it may be decided is desirable, is definitely a policy that needs careful discussion before being applied generally. It is relevant to these points that a subsequent prolonged multicentre study completed in 2009 led by Claire Wainwright of Brisbane comparing regular bronchoscopy with routine care failed to show a significant advantage for those who have regular bronchoscopies and bronchoalveolar lavages to monitor the microbiological state of their lower airways (Wainwright CE et al. JAMA 2011; 306:163-171. below [PubMed]).
Tom N Hilliard (fig 28) for this study at the Department of Paediatric Respiratory Medicine, Royal Brompton Hospital, London, UK. Subsequently Consultant Respiratory Paediatrician at Bristol Children’s Hospital
Homnick DN, Marks JH, Rubin BK. The effect of a first-generation antihistamine on sputum viscoelasticity in cystic fibrosis. J Aero Med 2007; 20:45-49. [PubMed]

Fig. 29 Douglas N Homnick CF Foundation
From this limited study, cryproheptadine hydrochloride (CH), a first-generation antihistamine, appears to have no adverse effects in sputum viscoelasticity or cough transportability in CF patients. This is reassuring as it was a widely held belief that in people with CF antihistamines”dried up” the sputum increasing the viscosity and should not be used.
Douglas N Homnick (fig. 29) is CF Center Director at the Division of Pediatric Pulmonology, Michigan State University, Kalamazoo Center for Medical Studies, Kalamazoo, Michigan 49008, USA.
Kempainen RR, Williams CB, Hazelwood A, Rubin BK, Milla CE. Comparison of high-frequency chest wall oscillation with differing waveforms for airway clearance in cystic fibrosis. Chest 2007; 132:1227-1232. [PubMed]

Fig. 30 Robert R Kempainen
med.umn.edu
Using the vest device, single-session sputum production is comparable with the sine and the triangular waveform high-frequency chest wall oscillation devices.There is a steady output of work on the various physiotherapy vest devices and although some seems to be related to companies competing for sales, it does seem to be a major advance particularly in convenience for all concerned.
— The use of the Vest is one of a number of major differences between treatment in the UK and USA where over 50% of people with CF use the Vest in contrast to the very few in the UK. Two obvious reasons are the cost of the equipment (approximately £10K in the UK) and the undoubted lack of enthusiasm of UK CF physiotherapists for the technique!!
Dr Robert R Kempainen (fig 30) is a pulmonologist in Minneapolis
Kennedy MP, Coakley RD, Donaldson SH, Aris RM, Hohneker K, Wedd JP, Knowles MR, Gilligan PH, Yankaskas JR. Burkholderia gladioli: five year experience in a cystic fibrosis and lung transplantation center. J Cyst Fibros 2007; 6:267-273. [PubMed]

Fig. 31 Marcus P Kennedy
leLocal.net
The impact of infection with Burkholderia gladioli in CF is unknown. Thirty-five patients were culture positive for B. gladioli, including 33 CF patients. Two-thirds of these isolates were susceptible to usual anti-Pseudomonal antibiotics. After acquisition, only 40% of the CF patients became chronically infected; chronic infection was associated with resistance to antibiotics on initial culture and failure of eradication after antibiotic therapy. The impact of acquisition of B. gladioli infection in chronic infection was variable. Three CF patients with chronic infection underwent lung transplantation. One post-transplant patient developed a B. gladioli mediastinal abscess, which was treated successfully. The authors concluded that the majority of patients who culture positive for B. gladioli have CF.
Apparently B. gladioliinfection is often transient and is compatible with satisfactory post-lung transplantation outcomes.Unfamiliar organisms are periodically isolated from people with CF and reports of experience such as this are very helpful in defining their importance for the clinician encountering the infection for the first time.
Marcus P Kennedy (fig. 31) at the Department of Pulmonary Medicine, Unit 403, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA. Subsequently Consultant Pulmonologist at Cork University Hospital, Ireland.
Kariya C, Leitner H, Min E, van Heeckeren C, van Heeckeren A, Day BJ. A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. Am J Physiol Lung C 2007; 292:L1590-7. [PubMed]
The cystic fibrosis transmembrane conductance regulator (CFTR) protein is the only known apical glutathione (GSH) transporter in the lung. The purpose of these studies was to determine whether oral GSH or glutathione disulfide (GSSG) treatment could increase GSH levels in lung epithelial lining fluid (ELF) and whether CFTR plays a role in this process. There was a rapid elevation in the GSH levels after oral GSH dosing and a selective increase in the reduced and active form of GSH in all lung compartments examined. Oral GSSG treatment (300 mg/kg) resulted in a smaller increase of GSH levels. To evaluate the role of CFTR in this process, Cftr knockout (KO) mice and gut-corrected Cftr KO-transgenic (Tg) mice were given an oral bolus dose of GSH (300 mg/kg) and compared with wild-type mice for changes in GSH levels in plasma, lung, ELF, and BAL cells. There was a twofold increase in plasma, a twofold increase in lung, a fivefold increase in ELF, and a threefold increase in BAL cell GSH levels at 60 min in wild-type mice; however, GSH levels only increased by 40% in the plasma, 60% in the lung, 50% in the ELF, and twofold in the BAL cells within the gut-corrected Cftr KO-Tg mice. No change in GSH levels was observed in the uncorrected Cftr KO mice.
Glutathione appears to be receiving increasing attention in the context of CF and these studies suggest that CFTR plays an important role in GSH uptake from the diet and transport processes in the lung (also see Bishop et al, 2005; Tirouvanziam et al, 2006; Childers M. 2007 – all above).
Dr Chirag Kariya is in the National Jewish Medical and Research Center, University of Colorado Health Sciences Center, Denver, Colorado, USA.
Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Resp Crit Care 2007; 176:1084-1089. [PubMed]
High-dose ibuprofen in a 4-year controlled trial slowed FEV(1) decline in young subjects with cystic fibrosis, but the effectiveness of ibuprofen has not been assessed in a large group of patients treated clinically with this therapy.
This study assessed the effect of ibuprofen therapy on FEV(1) decline in children and adolescents with cystic fibrosis, using observational data from the Cystic Fibrosis Foundation Patient Registry. The rate of decline in FEV(1) percent predicted over 2-7 years among patients age 6-17 years with FEV(1) > 60% predicted, and who were treated with ibuprofen (1,365), was compared with patients of similar age and disease severity who were not treated with this therapy (8,960). Multilevel repeated-measures mixed-regression models were used to estimate rates of decline, adjusting for characteristics and therapies that influenced FEV(1) decline. Adverse effects were compared among those treated versus not treated with ibuprofen.
FEV(1) declined less rapidly among patients treated with ibuprofen (difference, 0.60% predicted per year; 95% confidence interval, 0.31 to 0.89; P < 0.0001); a 29% reduction in slope based on an average decline of 2.08% predicted per year for patients not treated. Those treated with ibuprofen were more likely to have an episode of gastrointestinal bleeding requiring hospitalization, but the occurrence was rare in both groups (annual incidence, 0.37 vs. 0.14%; relative risk, 2.72; P < 0.001).
The authors concluded slower rates of FEV(1) decline are seen in children and adolescents with cystic fibrosis who are treated with ibuprofen. The authors consider the apparent benefits of ibuprofen therapy outweigh the small risk of gastrointestinal bleeding.
— This study from the CF Foundation Registry data confirms the slower decline of FEV1 in children treated with ibuprofen. However, despite a number of publications subsequent to Michael Konstan’s original study, the treatment never became popular and only 3.5% of patients on the CF Foundation Registry were taking the drug in 2010; it was not mentioned in 2011 list of treatments.
Khalid S, McGrowder D, Kemp M, Johnson P. The use of soluble transferrin receptor to assess iron deficiency in adults with cystic fibrosis. Clin Chim Acta 2007; 378:194-200. [PubMed]
Iron deficiency (ID) is common in cystic fibrosis (CF) and the soluble transferrin receptor (sTfR) is a sensitive, quantitative measurement of tissue iron deficiency. The study investigated the use of sTfR together with serum iron, transferrin saturation (TS) and serum ferritin, in assessing iron status in adult CF patients. The patient population consisted of 127 CF patients which consisted of 51 inpatients with infective exacerbation (IE) and 76 outpatients at the time of their annual review (AR). Serum sTfR was measured using a particle-enhanced immunoturbidimetric assay on the Beckman Coulter LX20.
Sixty five percent (65%) of CF patients in the IE group and 28% in the AR group had ID as determined by TS, but only 18% (IE group) and 20% (AR group) as determined by ferritin. Serum sTfR detected 20% in the IE group and 12% in the AR group. There was a significant correlation between C-reactive protein and TS (r=-0.56; P<0.01) but not with ferritin (r=0.22; P=0.380) in the IE group. The authors concluded that iron status of patients with CF can be accurately assessed by sTfR which is unaffected by the acute phase response and can be used in conjunction with serum ferritin.
Studies on iron and CF have appeared regularly since the 1960s and would continue to appear after this article! (Gifford A H et al. Pediatr Pulmonol 2011; 46:160-165.[PubMed]). The general view is that iron deficiency is common in CF and correlates with lung function and general health.
Dr Sabinha Khalid is at the Department of Clinical Biochemistry, Royal Brompton Hospital, Chelsea, London, UK, and Department of Pathology, University of the West Indies, Kingston, Jamaica.
Linde L, Boelz S, Nissim-Rafinia M, Oren YS, Wilschanski M, Yaacov Y, Virgilis D, Neu-Yilik G, Kulozik AE, Kerem E, Kerem B. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J Clin Invest 2007; 117:683-692. [PubMed]

Fig. 32 Liat Linde
bcf.technion.ac.il
Previously the authors found variable efficiency of readthrough in response to the aminoglycoside gentamicin among CF patients, all carrying the W1282X nonsense mutation. Here they demonstrate that there are patients in whom the level of CF transmembrane conductance regulator (CFTR) nonsense transcripts is markedly reduced, while in others it is significantly higher. Response to gentamicin was found only in patients with the higher level. They further investigated the possibility that the nonsense-mediated mRNA decay (NMD) might vary among cells and hence governs the level of nonsense transcripts available for readthrough. Their results demonstrate differences in NMD efficiency of CFTR transcripts carrying the W1282X mutation among different epithelial cell lines derived from the same tissue. Variability was also found for 5 physiologic NMD substrates, RPL3, SC35 1.6 kb, SC35 1.7 kb, ASNS, and CARS.
Importantly, these results demonstrate the existence of cells in which NMD of all transcripts was efficient and others in which the NMD was less efficient. Downregulation of NMD in cells carrying the W1282X mutation increased the level of CFTR nonsense transcripts and enhanced the CFTR chloride channel activity in response to gentamicin. The authors suggest that the efficiency of NMD might vary and hence have an important role in governing the response to treatments aiming to promote readthrough of PTCs in many genetic diseases. Appears to be one explanation for the conflicting results in trials of PTC 124.
Liat Linde (fig.32) is Head Technion at the Department of Genetics, Life Sciences Institute, The Hebrew University of Jerusalem, Jerusalem, Israel.
Liou TG. Adler FR. Cox DR. Cahill BC. Lung transplantation and survival in children with cystic fibrosis.[Erratum appears in N Engl J Med. 2008; Jul 31;359(5):e6]. N Eng J Med 2007; 357:2143-2152.[PubMed]. Free PMC article available

Fig. 33 Theodore G Liou
healthcare.utah.edu
The effects of lung transplantation on the survival and quality of life in children with cystic fibrosis are uncertain. The authors used data from the U.S. Cystic Fibrosis Foundation Patient Registry and from the Organ Procurement and Transplantation Network to identify children with cystic fibrosis who were on the waiting list for lung transplantation during the period from 1992 through 2002.
After detailed analysis (described in the full summary) a total of 248 of the 514 children on the waiting list underwent lung transplantation in the United States during the period from 1992 through 2002.They concluded that only 5 patients had a significant estimated benefit, 283 patients had a significant risk of harm, 102 patients had an insignificant benefit, and 124 patients had an insignificant risk of harm associated with lung transplantation [corrected]. The analyses estimated clearly improved survival for only 5 of 514 patients on the waiting list for lung transplantation.
Prolongation of life by means of lung transplantation should not be expected in children with cystic fibrosis. The authors concluded a prospective, randomized trial is needed to clarify whether and when patients derive a survival and quality-of-life benefit from lung transplantation.

Fig.34 Paul Aurora
gosh.nhs.uk
These conclusions were refuted by Paul Aurora (fig.34), transplant paediatrician at Great Ormond Street Hospital London where all the paediatric heart and lung transplants in children in the UK were performed. [PubMed]. He argues that the analysis is specific to the United States and predates the introduction of the lung allocation score, which has had a marked impact on how transplant organs are allocated in this country. It is likely that lung transplantation can extend life in both adults and children with cystic fibrosis, provided the procedure is correctly timed. Further development of the lung allocation score has the potential to increase the survival benefit from the procedure in the United States.[PubMed].
Theodore G Liou (fig.33) is Director of the Adult CF Center at the University of Utah, Professor of Internal Medicine and Adjunct Associate Professor of Pediatrics at the University of Utah. the Department of Internal Medicine, University of Utah, Salt Lake City, USA.
Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr 2007; 151:249-254. [PubMed]

Fig 35. Larry Lands mcgill.ca/expmed
The second major study on ibuprofen to assess the effectiveness and safety of high-dose ibuprofen when used as part of routine therapy in patients with CF, essentially to confirm the findings of Konstan (1995 above). 142 patients age 6 to 18 years with “mild” lung disease (FEV1 > 60% predicted) were randomized to receive either high-dose ibuprofen (70 subjects, 20 to 30 mg/kg/twice daily, adjusted to a peak serum concentration of 50 to 100 mug/mL) or placebo (72 subjects) for a 2-year period. The primary outcome was the annual rate of change in FEV1% predicted.
The patients in the high-dose ibuprofen group exhibited a significant reduction in the rate of decline of forced vital capacity percent predicted (0.07 +/- 0.51 vs -1.62 +/- 0.52; P = .03), but not of percentage predicted FEV1. The ibuprofen group also spent fewer days in hospital after adjusting for age (1.8 vs 4.1 days per year; P = .07). A total of 11 patients (4 in the ibuprofen group and 7 in the placebo group) withdrew due to adverse events. The authors concluded that high-dose ibuprofen has a significant effect on slowing the progression of lung disease in CF and generally is well tolerated.
Although Michael Konstan’s original study on ibuprofen showed some slowing of deterioration of respiratory function (Konstan et al, 1995 above), this was unimpressive and few clinicians prescribed the drug on a long term basis, also because of the side effects. Here Larry Lands re-evaluates the use of ibuprofen and although finding some positive effects it is most unlikely that many clinicians will prescribe the treatment in view of the very modest advantages and definite side effects. As already noted elsewhere ibuprofen treatment does appear to have an unexpected but favourable effect on nasal polyps (Lindstrom et al, 2007 below).
Dr Larry Lands’s (fig. 35) research studies at the Montreal Children’s Hospital are listed as modulation of inflammation in respiratory disease, with a particular interest in redox regulation. Other interests include nutritional intervention to enhance exercise and muscular ability.
LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ Jr. Cystic Fibrosis Foundation. Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr 2007; 151:85-89. [PubMed]

Fig. 36 Vicky LeGrys
med.unc.edu
The most recent guidelines from the CF Foundation. Similar guidelines have been published in the UK (Report from the Multi-Disciplinary Working Group. Anne Green Nov. 2003) and other countries so important is it that sweat tests are accurate to avoid the clinical disasters of mistaken diagnoses. Usually the mistakes are over-diagnosis due to the uncritical acceptance by the clinician of an false high sweat test (also Smalley et al, 1978 above; Shaw & Littlewood, 1987 above)
Professor Vicky A LeGrys (fig 36) is in the Division of Clinical Laboratory Science, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599-7145, USA.
Liu X, Luo M, Zhang L, Ding W, Yan Z Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Resp Cell Mol 2007; 36:313-323. [PubMed].

Fig. 37 Xiaoming Liu
medicine.iowa.edu
The development of effective therapies for cystic fibrosis (CF) requires animal models that can appropriately reproduce the human disease phenotype. CF mouse models have demonstrated cAMP-inducible, non-CF transmembrane conductance regulator (non-CFTR) chloride transport in conducting airway epithelia, and this property is thought to be responsible for the lack of a spontaneous CF-like phenotype in the lung.
Thus, an understanding of species diversity in airway epithelial electrolyte transport and CFTR function is critical to developing better models for CF.
Two species currently being used in attempts to develop better animal models of CF include the pig and ferret.
In the study reported here, the authors sought to comparatively characterize the bioelectric properties of in vitro polarized airway epithelia from human, mouse, pig and ferret grown at the air-liquid interface (ALI). Bioelectric properties analyzed include amiloride-sensitive Na(+) transport, 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS)-sensitive Cl(-) transport, and cAMP-sensitive Cl(-) transport. In addition, as an index for CFTR functional conservation, we evaluated the ability of four CFTR inhibitors, including glibenclamide, 5-nitro-2-(3-phenylpropyl-amino)-benzoic acid, CFTR (inh)-172, and CFTR(inh)-GlyH101, to block cAMP-mediated Cl(-) transport.Compared with human epithelia, pig epithelia demonstrated enhanced amiloride-sensitive Na(+) transport. In contrast, ferret epithelia exhibited significantly reduced DIDS-sensitive Cl(-) transport. Interestingly, although the four CFTR inhibitors effectively blocked cAMP-mediated Cl(-) secretion in human airway epithelia, each species tested demonstrated unique differences in its responsiveness to these inhibitors.
These findings suggest the existence of substantial species-specific differences at the level of the biology of airway epithelial electrolyte transport, and potentially also in terms of CFTR structure/function.
Xiaoming Liu (fig.37) is Research Professor in the Department of Anatomy, The Center for Gene Therapy, College of Medicine, The University of Iowa, Iowa City, Iowa 52242, USA.
Lindstrom DR, Conley SF, Splaingard ML, Gershan WM. Ibuprofen therapy and nasal polyposis in cystic fibrosis patients. J Otolaryngol 2007; 36:309-314.[PubMed]

Fig. 38 Richard Lindstrom drlindstroment.com
Twelve of 22 patients with CF were treated with high-dose ibuprofen therapy to benefit their pulmonary function. Twelve had nasal polyposis and all 12 patients had observed absence of nasal polyps at some point during their ibuprofen course; nasal polyps were present in five patients during ibuprofen therapy, and all resolved with increased ibuprofen doses. Polyps occurred in six of eight patients after ibuprofen therapy ceased. Five of the 12 patients required endoscopic sinus surgery for polyposis.
This is an interesting incidental finding that oral ibuprofen improves nasal polyposis. This could prove very helpful in patients with severe and recurring nasal polyposis which can be a very difficult problem.
D Richard Lindstrom(fig.38) is in the Department of Otolaryngology and Communication Sciences, Medical College of Wisconsin, Milwaukee, WI, USA.
Louis D, Duc ML, Reix P, Chazalette JP, Durieu I, Feigelson J, Bellon G. Partial splenectomy for portal hypertension in cystic fibrosis related liver disease. Pediatr Pulmonol 2007; 42:1173-80. [PubMed].

Fig 39. Jean-Pierre Chazalette
Over 20 years 19 patients aged 7-23 years underwent partial splenectomy (PS) for massive splenomegaly, hypersplenism and/or devere portal hypertension (PHT). In all but three cases, PHT and hypersplenism have improved for long periods. Noticeable improvement of hepatic tests occurred simultaneously. In all patients PS resolved abdominal discomfort. Fifteen patients are alive and a stabilization of the liver disease occurred with a follow-up of 1-20 years (mean 7.9). One patient died following respiratory insufficiency 10 years after PS although PHT was stable. Manifestations recurred in 2 patients 5 and 6 years after PS. In two patients, the course of the disease evolved to hepatic insufficiency without recurrence of PHT 3 and 8 years after PS. PS did not give the expected results in three cases only, in which PHT was not modified or reoccurred during the following year. No severe complication was observed. Early (three patients) or late (one patient) eventration required surgical procedure.
These results show that PS is a reliable and well-tolerated technique. Therefore, it is a therapeutic option for the management of PHT in CF patients with a preserved liver function. It can prevent and significantly delay a liver transplantation and its constraints. Information on the long term progress of patients followed by some of the authors for many years.

Fig. 40 Jean Fiegelson Author’s photo
Dr Jean Feigelson (fig.40) published his first paper on CF in 1960 (Feigelson J. Mem Soc Med Paris 1960;9:4-17. above) and was one of the pioneers of CF care in Europe.
Dr Jean-Pierre Chazalette (1939 – 2013) (fig. 39) specialised in CF treatment for 30 years. He provided specialist paediatric treatment from 1969 and in 1973 became Head of Services and Hospital Medicine at Renee Sabrin Hospital in Giens. Most recently he was at the Cystic Fibrosis Centre, Hopital Renee Sabran, Hyreres France. He was awarded the Legion d’Honneur in recognition of his work (fig.26).

Fig.41 Gabriel Bellon. Booknode
Dr Gabriel Bellon (fig. 41) is Professor of Paediatrics at l’université Claude-Bernard et pédiatre des hospices civils de Lyon. He has been involved in CF care and research and has published on many aspects of the condition and other respiratory disorders over the past 40 years.
Marcorelles P, Montier T, Gillet D, Lagarde N, Ferec C. Evolution of CFTR protein distribution in lung tissue from normal and CF human fetuses. Pediatr Pulmonol 2007; 42:1032-1040.[PubMed]
The distribution of CFTR protein progressively increased from 10 weeks of gestation (WG) to mid-gestation, but thereafter

Fig 42. Claude Ferec Le Telegramme
decreased until term. The CFTR protein was first detected in the cytoplasm of undifferentiated epithelial cells. Before mid-gestation, the immunostaining was strongly positive in bronchi, in sub-mucosal glands, and in lung parenchyma. Then, it became localized to the apical zone of the epithelial cells. This pattern correlated with differentiation during the second half of gestation. The main difference observed between normal and CF fetuses was a 3-week delay in detectability of the CFTR protein expression in the latter until 15 weeks of gestation. These results support the hypothesis of an early functional change. Abnormal fetal lung CFTR protein regulation could give rise to a predisposition to the post-natal inflammatory changes of the airways that characterize CF disease.
Professor Claude Ferec (fig. 42) is a leading European CF researcher based at the Laboratoire de Genetique Moleculaire, CHRU2 de Brest.
Massie J, Forbes R, Dusart D, Bankier A, Delatycki MB. Community-wide screening for cystic fibrosis carriers could replace newborn screening for the diagnosis of cystic fibrosis. J Paediatr Child H 2007; 43:721-723.[PubMed]

Fig. 43 John Massie
news.com.au
Most babies with cystic fibrosis (CF) are born to parents who did not know they were carriers until their baby was diagnosed with CF, usually by newborn screening. It is only after the birth of their first child with CF that couples are offered genetic counselling and reproductive choices. Most parents use this information for prenatal testing of subsequent pregnancies. With the high uptake of first trimester screening for Down syndrome (80% in Victoria, Australia) most couples have had screening during the CF affected pregnancy. Yet screening for CF carrier status is available, costs are similar to that for Down syndrome screening and CF carrier screening only ever needs to be done once. Waiting for couples to have a baby with CF before they are identified as carriers denies them choice. A national policy on CF carrier screening in Australia, and determination to equitably fund such a programme, is required.
— Although neonatal screening for CF has been introduced into many countries, there is now the means of identifying CF carrier parents which has been available for the last two decades. The feasibility of antenatal CF screening has been shown by the first studies from Edinburgh in the UK where such screening was pioneered by the late David Brock (Mennie ME et al. Lancet 1992; 340:214-216). [PubMed]). Also antenatal screening was recommended in a UK Health Technology Assessment in 1999 (Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J. Screening for cystic fibrosis. Health Technol Assess 1999;3(8). [PubMed]) and even agreed by the UK National Screening Committee but not introduced because of the cost (£50K – £100K for each affected fetus detected) and the lack of the necessary genetic counselling services.
It is inevitable that carrier screening will be introduced eventually for those that want it to provide all future parents with choice but this had not occured by 2023 in the UK. Screening for CF mutations on the NHS is currently available for relatives of people with CF if requested.
Professor John Massie (Fig. 43) is a leading paediatrician and researcher in Melbourne Australia.
Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, Spencer LT, Pilewski J, Waltz DA, Dorkin HL, Ferkol T, Pian M, Ramsey B, Carter BJ, Martin DB, Heald AE. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther 2007; 18:726-732. [PubMed]

Fig. 44 Richard Moss Author’s photo
One hundred and two subjects aged 12 years and older had either two aerosolized doses of 1×10(13) DNase-resistant particles of tgAAVCF (n=51) or matching placebo (n=51) administered 30 days apart. Although tgAAVCF was well tolerated, there were no significant differences in spirometric lung function over time, induced sputum biologic markers, or days of antibiotic use in either treatment group.So repeated doses of aerosolized tgAAVCF were safe and well tolerated, but did not result in significant improvement in lung function over time.
Because gene transfer is the simplest, most basic way to correct the underlying genetic defect due to many mutations, the authors considered that further research was warranted to develop an effective gene transfer agent for the treatment of CF.
Dr Richard B Moss (Fig.44) is chief of the Pediatric Pulmonary and Allergy Divisions, and Allergy-immunology and pulmonary fellowship training programs, at Stanford University. In 2017 he received the Cystic Fibrosis Champion Award, Cystic Fibrosis Research, Inc.
McLachlan G, Baker A, Tennant P, Gordon C, Vrettou C, Renwick L, Blundell R, Cheng SH, Scheule RK, Davies L, Painter H, Coles RL, Lawton AE, Marriott C, Gill DR, Hyde SC, Griesenbach U, Alton EW, Boyd AC, Porteous DJ, Collie DD. Optimizing aerosol gene delivery and expression in the ovine lung. Mol Ther: J Am Soc Gene Ther 2007; 15:348-354. [PubMed]

Fig. 45 Gerry McLachlan
research.ed.ac.uk
The authors have used the sheep as a large animal model for developing CF gene therapy protocols. They administered aerosolized gene transfer agents (GTAs) to the ovine lung in order to test the delivery, efficacy, and safety of GTAs using a clinically relevant nebulizer. Observing the behaviour of inhaled GTAs in airways of similar size to human lungs would allow them to move toward clinical studies with greater confidence. (also Davidson et al, 2006 above).The sheep is of more equivalent size than smaller laboratory animals for work leading to human gene therapy. Although there is not a CF-sheep yet, ovine and human CFTR can be identified and differentiated in the exposed tissues by immunological methods. In 2008 a CF pig would be reported (Rogers et al, Am J Physiol Lung Cell Mol Physiol 2008; 295:L240-L263)
Dr Gerry McLachlan (fig.45) is in the Medical Genetics Section, School of Molecular and Clinical Medicine, University of Edinburgh, Western General Hospital, Edinburgh, UK. Subsequently he became Group Leader at the Roslin Institute .
McKenzie SG, Chowdhury S, Strandvik B, Hodson ME. Investigators of the Epidemiologic Registry of Cystic Fibrosis. Dornase alfa is well tolerated: data from the epidemiologic registry of cystic fibrosis. Pediatr Pulmonol 2007; 42:928-937.

Fig 46. Shiela McKenzie
After closure of the Epidemiologic Registry of Cystic Fibrosis, a comprehensive safety analysis of dornase alfa (Pulmozyme) was performed. The results confirm the safety of dornase alfa in people with CF of all ages. Children under 5 years old appear to tolerate dornase alfa at least as well as older patients.There is an accumulating literature on the use of rhDNase in people with CF and most is favourable, confirming its beneficial effect and safety. In one large CF unit in the UK all children are given a trial on rhDNase as soon as inhalation therapy becomes feasible. Undoubtedly rhDNase is one of the more important treatment advances of the past 15 years (Shak et al, 1990 above; Fuchs et al, 1994 above).
Dr Sheila McKenzie (fig. 46), until recently of Hoffmann-La Roche ltd, has been involved with the development and introduction of Pulmozyme since the first clinical trials in the Nineties shortly after Pulmozyme was licensed. She was involved in the latter stages of the European phase IIIb study (published in 1998), the implementation (but not the original planning) of ERCF and the planning & implementation of the Quan mild patient study. She was the longest-serving member of Roche & Genentech staff to have worked with CF and has recently retired.
McCormick J. Conway SP. Mehta A. Paediatric Northern Score centile charts for the chest radiograph in cystic fibrosis. Clin Radiol 2007; 62:78-81.[PubMed]
All active patients for 2002 from the UK CF Database were analysed in 1-year cohorts from 0 to 18 years. Northern Score results from the annual review forms were used to construct centile lines for the 5th, 25th, 50th, 75th, 95th centiles. RESULTS: There were 1806 patients with recorded Northern Score data for 2002 (927 male patients, male:female ratio 1.05). The centile chart demonstrates a quasi-linear rise throughout childhood. A Northern Score in excess of age in years equates to >95th centile in school-aged CF patients.
This centile chart provides a disease-specific reference range for monitoring individual patients or for evaluating therapeutic change using the dominant chest radiograph scoring system in the UK. Patients, parents and clinicians may find these useful during the annual review process.The Northern Score referred to here was developed by the UK Northern CF Club members and is as follows – Conway SP, Pond MN, Bowler I, Smith DL, Simmonds EJ, Joannes DN, Hambleton G, Hiller EJ, Stableforth DE, Weller P, Littlewood JM. The chest radiograph in cystic fibrosis: a new scoring system compared with the Chrispin-Norman and Brasfield scores. Thorax 1994; 49:860-862. [PubMed]
Dr J McCormick is at the Respiratory Unit, Royal Hospital for Sick Children, Yorkhill NHS Trust, Glasgow, UK.
Nilsson E, Kollberg H, Johannesson M, Wejaker PE, Carlander D, Larsson A. More than 10 years’ continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report. J Med Food 2007; 10:375-378. [PubMed]

Fig. 47 Hans Kollberg author’s photo
Hans Kollberg (fig.47) and his colleagues in Sweden have used orally administered immunoglobulin Y (IgY)preparations for 10 years (Kollberg et al, 2003 above) and the patients treated with IgY had only 2.5 P. aeruginosa-positive sputum cultures per 100 months, and none became chronically infected. In the control group, 13.7 of the cultures per 100 months were positive for PA and 24% of patients became chronically infected.The product was recognised with Orphan Medicinal Product Designation by the EMeA in 2008 (see Kollberg H. Pediatr Pulmonol 2008; 43:982 below).
A European multicentre clinical trial was underway by 2012 and ended in 2016. The trial cost over 5 million euros. I could not find the results in a massive report https://cordis.europa.eu/project/id/261095/reporting#:~:text=IgY%20activity%20against%20PA%20is,total%20absence%20of%20adverse%20events.
Padman R, McColley SA, Miller DP, Konstan MW, Morgan WJ, Schechter MS, Ren CL, Wagener JS. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Infant care patterns at epidemiologic study of cystic fibrosis sites that achieve superior childhood lung function. Pediatrics 2007; 119:e531-7. [PubMed]
Previous analyses of the Epidemiologic Study of Cystic Fibrosis database revealed that sites with the highest average patient lung function monitor patients more frequently and treat with antibiotics more aggressively than those where average lung function is lowest. Infants at the upper quartile sites had more office and sick visits; more respiratory tract cultures; and more frequent use of intravenous antibiotics, oral corticosteroids, mast cell stabilisers (surprisingly sodium cromoglycate still seemed to be used in the USA), and mucolytics; but they received less chest physiotherapy, inhaled bronchodilators, oral nutritional supplements, and pancreatic enzymes. Data shows that both enrolment characteristics and infant care patterns are associated with lung function outcomes in later childhood. The authors suggested that pulmonary function of older children may be improved through specific interventions during the first 3 years of life.
— The conclusions of this important analysis are likely to be quite obvious to those clinicians to whom have been referred many children permanently damaged before being eventually diagnosed as having cystic fibrosis and appropriately treated. Also they will have noticed the contrast with those infants diagnosed and treated early after newborn screening. However, this excellent data is very welcome to convince those who are responsible for funding CF care and neonatal screening programmes and who cannot, or refuse to, draw on clinical experience.
It is interesting that in all studies of this nature over the years the more frequent use of IV antibiotics and more frequent follow-up visits are always features in the history of those with better results (Woods & Piazza, 1988 above).
Dr Raj Padman a pediatric pulmonologist at the Department of Pediatrics, Alfred I. duPont Hospital for Children, Nemours Children’s Clinic, Wilmington, DE 19899, USA.
Pall H, Zielenski J, Jonas MM, DaSilva DA, Potvin KM, Yuan XW, Huang Q, Freedman SD. Primary sclerosing cholangitis in childhood is associated with abnormalities in cystic fibrosis-mediated chloride channel function. J Pediatr 2007; 151:255-259. [PubMed]

Fig. 48 Harpreet Pall LinkedIn
Twenty children with primary sclerosing cholangitis (PSC) diagnosed in childhood had sweat chloride concentration, nasal transmembrane potential difference, and extensive genetic analysis of the CFTR gene. Disease control subjects consisted of 14 patients with inflammatory bowel disease alone and no liver disease.In the PSC group, CFTR chloride channel function was markedly diminished at -8.6 +/- 8.2 mV (reference range: -24.6 +/- 10.4 mV). In contrast, disease control subjects had normal function, at -17.8 +/- 9.7 mV (P = .008). Sweat chloride concentration in subjects with PSC was greater than in disease control subjects (20.8 +/- 3.4 mmol/L vs 12.0 +/- 1.6 mmol/L, P = .045).
Comprehensive CFTR genotyping revealed that 5 of 19 (26.3%) subjects with PSC had a CFTR mutation or variant, compared with 6 of 14 (42.9%) disease control subjects. The authors concluded there is a high prevalence of CFTR-mediated ion transport dysfunction in subjects with childhood PSC.
The presence of one CF mutation in 26.3% of people with primary sclerosing cholangitis is similar to the increased frequency of CF mutations in pancreatitis. This is the first report of this association with sclerosing cholangitis. It is not clear why the CF mutations are so frequent in the control group and this is not explained in the paper.
Dr Harpreet Pall (fig 48) is in the Division of Pediatric Gastroenterology, Children’s Hospital Boston, Harvard Medical School, Boston, MA 02115, USA. Subsequently Chair and Professor of the Department of Pediatrics at Hackensack Meridian School of Medicine and K. Hovnanian Children’s Hospital at Jersey Shore University Medical Center.
Poschet JF, Timmins GS, Taylor-Cousar JL, Ornatowski W, Fazio J, Perkett E, Wilson KR, Yu HD, de Jonge HR, Deretic V. Pharmacological modulation of cGMP levels by phosphodiesterase 5 inhibitors as a therapeutic strategy for treatment of respiratory pathology in cystic fibrosis. Am J Physiol Lung Cellular & Molecular Physiology 2007; 293:L712-719. [PubMed]

Fig. 49 Jens Poschet LinkedIn
Pharmacological inhibition of phosphodiesterase 5 in tissues ex vivo or in animal models improved transepithelial currents across nasal mucosae from transgenic F508del Cftr (tm1Eur) mice and reduced neutrophil infiltration on bacterial aerosol challenge in Pseudomonas aeruginosa-susceptible DBA/2 mice.
The authors consider the present findings define phosphodiesterase 5 as a specific target for correcting a number of previously disconnected defects in the CF respiratory tract, now linked through this study and suggest that phosphodiesterase 5 inhibition provides an opportunity for simultaneous and concerted correction of seemingly disparate complications in cystic fibrosis.The potential for phosphodiesterase type 5 inhibitors as a treatment for CF continue to appear but as yet no clinical trial has been reported although interest continued (see Topics).
Dr Jens Poschet (fig.49) is at the Department of Molecular Genetics and Microbiology, University of New Mexico School of Medicine, Albuquerque, New Mexico 87131, USA.
Ratjen F, Walter H, Haug M, Meisner C, Grasemann H, Doring G. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr Pulmonol 2007; 42:249-255. [PubMed]
The authors used 1,791 serum samples from 375 European CF patients with known respiratory microbiology status to define titers of P. aeruginosa antibodies directed against alkaline protease (AP), elastase (ELA), and exotoxin A (ExoA). Pseudomonas antibody titers were also measured in a separate cohort of 56 patients undergoing antibiotic treatment for eradication of P. aeruginosa. At a specificity of 97.5%, the sensitivity was highest for antibodies against AP (85.4%), followed by ELA (76.2%) and ExoA (72.0%). AP, ELA, or ExoA antibody titers were significantly higher (P < 0.001) in patients chronically infected with P. aeruginosa compared to patients with negative cultures. The sensitivity of the combined three ELISAs was higher than that for any single ELISA alone. Based on the newly defined cut-off levels, positive serum antibody titers against at least one of the three antigens were present in 43% of patients with new onset of P. aeruginosa infection.Longitudinal assessment of antibody titers assessed before and after inhaled antibiotic therapy in patients with first P. aeruginosa isolation showed a significant decrease in antibody titers against AP and ExoA in patients clearing P. aeruginosa infection, whereas titers increased in patients in whom antibiotic therapy failed to eradicate the organism. Antibody testing against AP, ELA, and ExoA offers high sensitivity and specificity for the presence of P. aeruginosa in respiratory cultures of CF patients. Although serum antibody titers are on average low at the time of first P. aeruginosa isolation from respiratory specimens, they may be useful to monitor response to therapy. However, because variability between patients is considerable, treatment decisions should not be based on P. aeruginosa antibody levels alone.
— Many CF centres such as Copenhagen and Leeds have been using Pseudomonas antibodies for over 25 years and finding them a very useful, indeed they are now regarded as an essential aid, for patient management. The main value in paediatric clinics where most children with CF are not chronically infected by P. aeruginosa is for periodic reassurance that they are not infected by P. aeruginosa. Should they be antibody positive, in the presence of a negative respiratory cultures, a search is made for the organism if necessary including by bronchoalveolar lavage. In patients who are chronically infected with P. aeruginosa a rising titre is an indication for more aggressive antibiotic treatment.
Rideout K. Evaluation of a PNP (pediatric nurse practitioner) care coordinator model for hospitalized children, adolescents, and young adults with cystic fibrosis. Pediatr Nurs 2007; 33:29-35; quiz 35-6. [PubMed]

Fig. 50. Kathy Rideout son.rochester.edu
The purpose of this study from Rochester was to evaluate a new model for delivery of care to hospitalized children, adolescents, and young adults with cystic fibrosis (CF) admitted to the adolescent unit at Golisano’s Children’s Hospital at Strong. The specific aim of this study was to test the hypothesis that children, adolescents, and young adults with CF who were hospitalized under the new model for care delivery would have better experiences and clinical outcomes than they had during previous hospitalizations prior to the implementation of the new model.An inpatient Pediatric Nurse Practitioner Care Coordinator (PNP-CC) for CF patients admitted to the hospital can reduce the time for ancillary service consultations, reduce LOS, and improve patient and health care provider satisfaction.
— The value of CF Nurse Specialists is now undisputed for over 30 years and it is no surprise that concentrating the efforts of such a person on the inpatient care of young people with CF would lead to better results and increased user satisfaction. This has been increasingly true as the technical aspects of CF care have have become more specialised and complicated. A frequent complaint in the UK Health Service is the lack of continuity and lack of expertise on the part of both medical and nursing staff. Fortunately, in the UK an exception is the specialised units such as CF centres. To have a CF Nurse Specialist working on the ward would undoubtedly improve care in many units.
Kathy Rideout (fig.50) is Professor of Clinical Nursing at the University of Rochester School of Nursing.
Rowe SM, Varga K, Rab A, Bebok Z, Byram K, Li Y, Sorscher EJ, Clancy JP. Restoration of W1282X CFTR activity by enhanced expression. Am J Resp Cell Mol Biol 2007; 37:347-356. [PubMed]

Fig. 51 Steven M. Rowe
Various aminoglycosides induce “translational readthrough” of premature stop codons and have been shown to restore full-length functional protein in a number of preclinical and clinical settings. The authors studied two well-described premature termination codons found in the distal open reading frame of CFTR, W1282X and R1162X, expressed in polarizing and non-polarizing cells.Their findings indicate that W1282X CFTR-expressing cells demonstrate significantly greater CFTR activity when over-expressed compared with R1162X CFTR cells, even when truncated protein is the predominant form. In addition, their results show that the combination of stimulated expression and stop codon suppression produces additive effects on CFTR-mediated ion transport. These findings are considered to provide evidence that W1282X CFTR exhibits membrane localization and retained chloride channel function after enhanced expression, and suggest that patients harbouring this mutation may be more susceptible to CFTR rescue.
Steven Rowe (fig.51) is professor in the Department of Medicine, Gregory Fleming James Cystic Fibrosis Research Center, University of Alabama at Birmingham, Birmingham, Alabama
Sermet-Gaudelus I, Renouil M, Fajac A, Bidou L, Parbaille B, Pierrot S, Davy N, Bismuth E, Reinert P, Lenoir G. Les BMC Medicine 2007; 5:5. In vitro prediction of stop-codon suppression by intravenous gentamicin in patients with cystic fibrosis: a pilot study. [PubMed]

Fig. 52 Isabelle Sermet-Gaudelus MyScienceWork
A pilot study was conducted to determine whether intravenous gentamicin suppresses stop codons in CF patients and whether it has clinical benefits. A dual gene reporter system was used to determine the gentamicin-induced readthrough level of the most frequent stop mutations within the CFTR in the French population. The authors investigated readthrough efficiency in response to 10 mg/kg once-daily intravenous gentamicin perfusions in patients with and without stop mutations. Respiratory function, sweat chloride concentration, nasal potential difference (NPD) and CFTR expression in nasal epithelial cells were measured at baseline and after 15 days of treatment.
After in vitro gentamicin incubation, the readthrough efficiency for the Y122X mutation was at least five times higher than that for G542X, R1162X, and W1282X. In six of the nine patients with the Y122X mutation, CFTR immunodetection showed protein at the membrane of the nasal epithelial cells and the CFTR-dependent Cl- secretion in NPD measurements increased significantly. Respiratory status also improved in these patients, irrespective of the gentamicin sensitivity of the bacteria present in the sputum. Mean sweat chloride concentration decreased significantly and normalised in two patients. Clinical status, NPD and sweat Cl- values did not change in the Y122X patients with no protein expression, in patients with the other stop mutations investigated in vitro and those without stop mutations.
The authors concluded suppression of stop mutations in the CFTR gene with parenteral gentamicin can be predicted in vitro and is associated with clinical benefit and significant modification of the CFTR-mediated Cl- transport in nasal and sweat gland epithelium
Isabelle Sermet-Gaudelus (Fig 52) is professor of Pediatrics and Head of the Pediatric CF Centre. at the Centre de Ressources et de Compétence en Mucoviscidose, Hôpital Necker-Enfants Malades, AP-HP, Paris, France.
Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F. German Cystic Fibrosis Growth Hormone Study Group. A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the metabolic and respiratory effects of growth hormone in children with cystic fibrosis. Pediatrics 2007; 119:e1230-1238. [PubMed]

Fig. 53 Dirk Schnabel
spz.charite.de
Another multicenter, randomised, placebo-controlled, double-blind study to assess the efficacy and safety of 2 dosages of growth hormone in cystic fibrosis. Sixty-three dystrophic patients with cystic fibrosis were randomly assigned for 24 weeks to 1 of 3 treatment arms followed by an open treatment period of 24 weeks. Height, growth velocity, and growth factors increased significantly in both treatment groups, whereas weight gain did not differ between the growth hormone groups and placebo. Maximal oxygen uptake during peak exercise increased significantly in treated patients.
The authors concluded that these data suggest that in the group investigated, growth hormone therapy was well tolerated and had positive metabolic effects but did not result in short-term improvement of lung function in patients with cystic fibrosis.
— It is unlikely that many CF clinicians would advise growth hormone therapy except perhaps in exceptional circumstances; also it is hoped that with present day treatment (2023) there will be fewer dystrophic children who would benefit from this form of treatment which involves daily subcutaneous injections and costs up to £15K per year (see also Hardin et al, 2001 above; Hardin et al, 2006 above; Staveley MS et al. A multi-center controlled trial of growth hormone treatment in children with cystic fibrosis. Pediatr Pulmonol 2012; 47:252-263. below).
Dirk Schnabel (fig. 53) is at the Department of Pediatric Endocrinology and Diabetology, Children’s Hospital, Charite, Berlin, Germany.
Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A, on behalf of the UK Cystic Fibrosis Database Steering Committee. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics 2007; 119:19-28. [PubMed]

Fig 54. Erika Sims
Flu care
Another important paper by Erika Sims (fig. 40) using data from the UK CF database then based in Dundee. Homozygous DF508 infants aged one to 10 years born between 2000 and 2002 were stratified into newborn screened, early diagnosed or late diagnosed. Newborn screening was associated with greater height Z scores, higher Shwachman-Kulczycki scores, lower likelihood of a height less than tenth centile and fewer long term therapies compared with late clinically diagnosed patients.
This is hard data further supporting the case for newborn screening which the UK National Screening Committee were so reluctant to agree to until the Wisconsin paper of 2001 showed a definite long term advantage in terms of growth (Farrell et al, Pediatrics 2001; 107:1-13 above).
– Unfortunately it is still true that the need for “evidence-based medicine” delayed the introduction of newborn CF screening in the UK by a decade when the need was so blatantly obvious to most experienced paediatricians and families! Apologies for mentioning this more than once but the delay in introducing national neonatal CF screening had a disastrous effect on the ultimate survival of a minority of children with CF who were unfortunate to be born in a non-screening area of the UK and not diagnosed until their chests were irretrievably damaged.
Dr Erika Sims (fig 54) subsequently became Operations Manager Norwich Medical School; Member, Norwich Clinical Trials Unit; Member, Public Health and Health Services Research.
Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla C, Moran A. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007; 30:1056-1061.[PubMed]
Fig. 55. Sarah Jane Schwarzenberg M Health Fairview
The incidence of cystic fibrosis-related diabetes (CFRD) and the prevalence of diabetic microvascular complications were determined at the University of Minnesota. During 1990-2005, 775 patients aged 6 years and older were followed. CFRD was diagnosed in 285 subjects (52% female), 64% of whom had fasting hyperglycaemia. No subject with CFRD without fasting hyperglycaemia had retinopathy or abnormal urine albumin/creatinine ratio.In CFRD subjects with fasting hyperglycaemia and diabetes for 10 years or longer, 14% had microalbuminuria and 16% had retinopathy. Autonomic neuropathy and gastrointestinal symptoms each were seen in 52% and somatic abnormalities in 22% of patients with or without fasting hyperglycaemia.
The authors concluded that diabetic microvascular complications occur in CFRD, although the prevalence of retinopathy and nephropathy appears to be less than that found in other forms of diabetes. They advised that annual complication screening should occur after known diabetes duration of 5 years in patients with CFRD with fasting hyperglycaemia.It is likely that CFRD will prove to be an increasingly important complication as the age of the adult CF population increases as will its complications as described here (Also Andersen et al, 2006 above).
Sarah Jane Schwarzenberg (Fig. 55) is Professor of Gastroenterology, Hepatology and Nutrition in the Department of Pediatrics, University of Minnesota.
Sousa M, Ousingsawat J, Seitz R, Puntheeranurak S, Regalado A, Schmidt A, Grego T, Jansakul C, Amaral MD, Schreiber R, Kunzelmann K. An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: A potential treatment for cystic fibrosis. Mol Pharmacol 2007; 71:366-376.
Functional assays by Ussing chamber, patch-clamping, double-electrode voltage-clamp and Ca2+ imaging demonstrate activation of Cl- secretion and inhibition of Na+ absorption by herbal extracts of the common Thai medicinal euphorbiaceous plant Phyllanthus acidus. Apparently Phyllanthus acidus corrects defective electrolyte transport in CF airways by parallel mechanisms including 1) increasing the intracellular levels of second messengers cAMP and Ca2+, thereby activating Ca2+-dependent Cl- channels and residual CFTR-Cl- conductance; 2) stimulating basolateral K+ channels; 3) redistributing cellular localization of CFTR; 4) directly activating CFTR; and 5) inhibiting ENaC through activation of CFTR.The authors, from Germany, suggest that these combinatorial effects on epithelial transport may provide a novel complementary treatment for the CF lung disease.
However, no further publications related to CF had appeared by 2023 although there were a number of publications relating to protection from induced hepatotoxicity and a review of genus Phyllanthus ( https://pubmed.ncbi.nlm.nih.gov/27200104/)
Marisa Sousa is at the Institut für Physiologie, Universität Regensburg, Universitätsstrasse 31, D-93053 Regensburg, Germany.
Subbarao P, Balkovec S, Solomon M, Ratjen F. Pilot study of safety and tolerability of inhaled hypertonic saline in infants with cystic fibrosis. Pediatr Pulmonol 2007; 42:471-476. [PubMed]

Fig. 56. Padmaja Subbarao physiology.utoronto.ca
A prospective trial of inhaled hypertonic 7% saline in 13 infants with CF aged 25-140 weeks who were undergoing specialised infant respiratory function tests (standardized raised volume rapid thoracoabdominal compression tests). In this pilot study, inhalation of hypertonic 7% saline by mask was well tolerated by the CF infants.These results support a study of the efficacy of hypertonic saline in this age group which is proposed by the authors and which started in 2008.
Subsequent studies followed confirming the safety and tolerability and in 2023 nebulised hypertonic saline received grudging support from Cochrane reviewers https://pubmed.ncbi.nlm.nih.gov/37319354/
— Hypertonic saline was first shown to improve mucociliary clearance in CF by Robinson M, Regnis JA, Bailey DL, King M, Bautovich GJ, Bye PT. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am J Respir Crit Care Med 1996; 153:1503-1509. [PubMed]).
Dr Padmaja Subbarao (fig.56) is a senior scientist in the Division of Pediatric Respiratory Medicine, The Hospital for Sick Children, University of Toronto, Toronto, Canada.
Sims EJ, Mugford M, Clark A, Aitken D, McCormick J, Mehta G, Mehta A. UK Cystic Fibrosis Database Steering Committee. Economic implications of newborn screening for cystic fibrosis: a cost of illness retrospective cohort study. Lancet 2007; 369(9568):1187-1195. [PubMed]
This study estimated the potential savings in treatment costs attributable to newborn screening using the UK Cystic Fibrosis Database. The yearly costs of long-term therapies and intravenous antibiotics for 184 patients who were diagnosed as a result of screening as newborn babies, and 950 patients who were clinically diagnosed aged 1-9 years in 2002 were compared. The cost of therapy for patients diagnosed by newborn screening was highly significantly lower than equivalent therapies for clinically diagnosed patients: mean ($7228 vs $12 008) and median ($352 vs $2442). Clinical, social, and economic evidence suggests that universal newborn screening programmes for cystic fibrosis should be adopted internationally.

Fig 54 Erika Sims
Flucare
This is one of a number of important and practically useful papers from the UK Database during the time it was based in Dundee. Dr Erika Sims (fig. 54) had a lead role in writing the reports. The UK Database was replaced by the UK CF Registry in 2007. The important practical message of this study was that although there may be small differences in the clinical condition of screened and non-screened patients for many years through childhood, the cost of keeping many of the non-screened, late diagnosed patients at a similar level to the screened patients was much greater
Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A. United Kingdom Cystic Fibrosis Database Steering Committee. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics 2007; 119:19-28. [PubMed]
In a cross-sectional analysis of cohorts retrospectively ascertained, patients who were homozygous deltaF508 with cystic fibrosis, attending specialist cystic fibrosis centres, and 1 to 10 years of age between 2000 and 2002 were identified from the United Kingdom Cystic Fibrosis Database and stratified into newborn-screened, early-clinically diagnosed, or late-clinically diagnosed cohorts. Newborn screening was associated with higher height Z score, higher Shwachman-Kulczycki score, lower likelihood of height <10th percentile, and fewer long-term therapies compared with late-clinically diagnosed patients.Further hard evidence in support of neonatal CF screening using data from the UK database.

Fig. 58. Gita Mehta

Fig. 57. Anil Mehta
The UK Database was developed by Dr Anil Mehta (fig. 41) and Mrs Gita Mehta (fig. 42) in Dundee for the UK CF Trust. The data formed the basis of a number of very important papers.
From 2007 the patient data was transferred to the CF Trust Registry – a system based on the CF Foundation Port CF system.
Veenstra DL, Harris J, Gibson RL, Rosenfeld M, Burke W, Watts C. Pharmacogenomic testing to prevent aminoglycoside-induced hearing loss in cystic fibrosis patients: potential impact on clinical, patient, and economic outcomes. Genet Med 2007; 9:695-704. [PubMed]

Fig 59 David Veenstra sop.washington.edu
Recently, a genetic test to identify patients with a mitochondrial mutation (A1555G) that may predispose patients aminoglycoside induced deafness has become available. Although the A1555G variant is very rare, it seems to confer a high risk of severe hearing loss in patients exposed to aminoglycosides. The authors calculated that A1555G testing decreased the risk of severe aminoglycoside-induced hearing loss by 0.12% in the cystic fibrosis population. The results of their analysis suggest that there are significant data gaps and uncertainty in the outcomes with A1555G testing, but it is not likely cost-effective, and could lead to worse patient outcomes due to avoidance of first-line therapy in the >95% of patients who are false-positives. Additional research is needed before pharmacogenetic testing for the A1555G mitochondrial mutation can be recommended, even in a population with a high likelihood of exposure to aminoglycosides.
The full methodology is described in this paper from existing published data and the conclusion was that the denial of aminoglycoside therapy would overall worsen the prognosis. However, it is important to stress that repeated courses of gentamicin are avoided in people with CF.
Dr David Veenstra (fig. 59) is Professor in the School of Pharmacy University of Washington.
Verhaeghe C, Delbecque K, de Leval L, Oury C, Bours V. Early inflammation in the airways of a cystic fibrosis foetus. J Cyst Fibros 2007; 6:304-308. [PubMed]

Fig. 60. Caroline Verhaeghe Actu.fr
In people with CF, inflammation is often considered to be secondary to chronic infections. In the present study, there were increased levels of pro-inflammatory proteins in the lungs of a CF fetus compared to the lungs of two non-CF fetuses. The authors consider their findings suggest the existence of an early intrinsic pro-inflammatory state in cystic fibrosis airways.It seems clear that inflammation occurs in CF and the airways react excessively when infection occurs. In this study the implication is that infection is not required to initiate inflammation. Probably difficult to come to any conclusions on this one report. However this observation could be important as it has been suggested by Larson et al that intrauterine treatment would be ideal for the CF fetus.
Caroline Verhaeghe (fig. 60) is in the Department of Human Genetics, Center for Biomedical Integrative Genoproteomics (CBIG), University of Liege, CHU Sart-Tilman B35, B-4000 Liege, Belgium.
Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem 2007; 282:4533-4544. [PubMed]
Although CFTR activation by curcumin required neither ATP binding nor heterodimerization of the two NBDs, it was strongly dependent on prior channel phosphorylation by protein kinase A. Curcumin is a useful functional probe of CFTR gating that opens mutant channels by circumventing the normal requirements for ATP binding and NBD heterodimerization. The phosphorylation dependence of curcumin activation indicates that the R domain can modulate channel opening without affecting ATP binding to the NBDs or their heterodimerization.
Curcumin, despite its disappointing clinical results after initial encouraging mouse studies (Egan et al, 2004 above) does seem to have some important effects and studies on curcumin continued.
Wei Wang is in the Department of Physiology and Biophysics and the Gregory Fleming James Cystic Fibrosis Research Center, University of Alabama at Birmingham, Birmingham, Alabama 35294.
Wiedemann B, Paul KD, Stern M, Wagner TO, Hirche TO. German CFQA Group. Evaluation of body mass index percentiles for assessment of malnutrition in children with cystic fibrosis. Eur J Clin Nutr 2007; 61:759-768.[PubMed].
To compare the performance of recently released body mass index percentiles (BMIp) with standard anthropometric indexes, including height-for-age percentile (HAP), weight-for-age percentile (WAP) and percent ideal body weight (%IBW), as measures for nutritional failure in children with cystic fibrosis (CF). Cross-sectional analysis of growth and lung function data from 4577 children with CF reported to the German CF quality assurance (CFQA) project from 1995 to 2004 were used.The authors found that BMIp predicts nutritional failure more sensitively and accurately than conventional anthropometric indexes, at least in children with CF. Consequently, less CF children were identified with nutritional failure according to %IBW method (male 20.5%; female 22.7%) compared with BMIp method (male 30.4%; female 28.7%). The clinical relevance of these findings was confirmed by stronger correlation of BMIp with impaired %forced expiratory volume/s, a marker for disease progression in CF. Screening of CF patients by BMIp could provide an early warning sign and allow for timely therapeutic intervention. This paper confirms the increased sensitivity of the Body Mass Index Percentile (BMIp) with the previously used anthropomorphic values for children with CF.
B. Wiedemann is in the Department of Medical Informatics and Biometrics, Technical University Dresden, Dresden, Germany.
Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007; 447 (7140):87-91. [PubMed]

Fig. 61 Ellen Welch ptcbio.com
PTC124 selectively induces ribosomal readthrough of premature but not normal termination codonsC124 activity promoted dystrophin production in primary muscle cells from humans and mdx mice expressing dystrophin nonsense alleles, and rescued striated muscle function in mdx mice within 2-8 weeks of drug exposure.The drug may have broad clinical potential for the treatment of a large group of genetic disorders with limited or no therapeutic options including muscular dystrophy and cystic fibrosis. (also Kerem E et al. Effectiveness of PTC 124 treatment for cystic fibrosis caused by nonsense mutations: a prospective phase 11 trial. Lancet 2008; 372:719-727.[PubMed] below).
Dr Ellen M Welch (fig. 61) is chief scientific officer at PTC Therapeutics, 100 Corporate Court, South Plainfield, New Jersey 07080, USA.
[Some of the significant papers relating to PTC 124 are grouped together in Topics in New Treatments]
Yu ZY, McKay K, van Asperen P, Zheng M, Fleming J, Ginn SL, Kizana E, Latham M, Feneley MP, Kirkland PD, Rowe PB, Lumbers ER, Alexander IE. Lentivirus vector-mediated gene transfer to the developing bronchiolar airway epithelium in the fetal lamb. J Gene Med 2007; 9:429-439. [PubMed]

Fig 62. Ian Alexander
LinkedIn
Targeting the developing airway epithelium during fetal life offers the prospect of circumventing some of the problems with gene therapy. The authors investigated vesicular stomatitis virus glycoprotein (VSVg)-pseudotyped HIV-1-derived lentivirus vector-mediated gene transfer to the airway epithelium of mid-gestation fetal lambs, both in vitro and in vivo. Even during the early pseudoglandular and canalicular phases of lung development, occurring through mid-gestation, the proximal bronchial airway epithelium was relatively mature and highly resistant to lentivirus-mediated transduction. In contrast, the more distal bronchiolar airway epithelium was relatively permissive for transduction although the absolute levels achieved remained low.
There had been repeated suggestions that fetal gene therapy would be necessary (Larson et al, 1997; Cohen & Larson, 2006 above) although the work on which these suggestions were based was not repeatable in a careful UK study (Buckley et al, 2008 below). Also it is very unlikely that fetal gene therapy would ever be advisable or indeed approved by the regulatory authorities.
Dr Ze-Yan Y is in the Department of Respiratory Medicine, The Children’s Hospital at Westmead, Westmead, Sydney, NSW, Australia.
Prof. Ian Alexander (fig. 62) is Head, Gene Therapy Research Unit, Sydney Children’s Hospitals Network and Children’s Medical Research Institute of Respiratory Medicine, The Children’s Hospital at Westmead, Westmead, Sydney, NSW, Australia.

