Liver disease
Introduction
As the mean age of survival for patients with cystic fibrosis (CF) continues to improve, the diagnosis and treatment of CF liver disease (CFLD) has become a very relevant clinical issue. Cystic fibrosis liver disease is the third most frequent cause of death in CF after respiratory and transplantation complications and accounts for 2.3% of all mortality (Cystic Fibrosis Foundation, 2002). Liver disease is the initial diagnostic finding in 1.5% of patients, suggesting that all patients with unexplained cirrhosis should have a sweat test as part of their diagnostic evaluation (Collardeau-Frachon et al, 2007).
The only potentially curative treatment for liver failure (the development of jaundice, ascites and encephalopathy) is transplantation. However, even in established cases of cirrhosis, serum liver enzymes may be completely normal. Early detection of liver disease is thus of vital importance to allow treatment to prevent or delay its progression. Due to the increased and widespread use of laboratory tests, and clinical and ultrasound examination, more patients with CF are now diagnosed with only minor degrees of liver involvement.
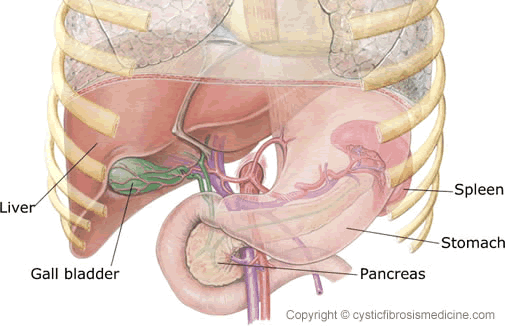
Figure 1. Anatomy of the liver, spleen, gall bladder and pancreas
Cystic fibrosis liver disease is due to impaired secretory function of the biliary epithelium and therefore absent or dysfunctional CFTR protein is fundamental to its pathogenesis (Colombo et al, 1999). The CFTR protein is expressed exclusively at the apical domain of epithelial cells lining the intra- and extrahepatic bile ducts and gall bladder (Cohn et al, 1993; Kinnman et al, 2000). It is not expressed in hepatocytes or other cells of the liver and its main role is to participate in the first stage of ductal secretion. It is thought that the primary chloride channel defect results in dehydrated, inspissated secretions that plug and obstruct the intrahepatic bile ducts and initiate a progressive periportal fibrosis. This theory, however, fails to explain why only a third of patients develop clinically significant liver disease and why CFLD shows such variable severity despite all patients having absent or abnormal CFTR function.
This variability might be explained by non-CFTR modifying genetic or environmental factors that determine whether or not liver changes will progress to clinical significance in any given case. There has been much research in this area. The existence of modifier genes has been suggested by sibling studies (Castaldo et al, 2001). It seems likely that multiple genes are involved. Those under current study include alpha-1-antitrypsin, transforming growth factor, and mannose binding lectin (Lewindon et al, 2002; Salvatore et al, 2002). The presence of mutations or variants in all three genes has been associated with the greatest risk of developing CFLD with an odds ratio of 11.4 (Friedman et al, 2002).
The role of glutathione S-transferase has also been implicated in the development of CFLD (Henrion-Caude et al, 2002). Cystic fibrosis transmembrane conductance regulator modulates glutathione transport and thus CFTR dysfunction creates an imbalance in antioxidant defences. The glutathione genotype is expressed in the biliary epithelium in significantly higher amounts in patients with CF liver disease. Amongst the youngest patients with CF this genotype has been associated with an eight fold increase in the risk of liver disease. Identification of this and other polymorphisms may have prognostic value and prompt early treatment in patients at increased risk of liver disease.
Other factors, intrinsic to the patient may determine whether CFLD will develop. Several studies cite a higher risk in male patients, in those with a history of meconium ileus (5-fold increase) and in those with a severe CFTR mutation (Colombo et al, 1994; Colombo et al, 2002; Lamireau et al, 2004). Although not universal (Slieker et al, 2003), meconium ileus has been recognised as a risk factor in most studies. Possible explanations include prolonged bowel obstruction which may influence biliary secretion and cause plugging in the biliary tree, extensive surgical management and total parenteral nutrition (Shapira et al, 1999). Smith et al found elevated endogenous biliary concentrations of ursodeoxycholic acid (UDCA) in patients with CF but without CFLD, compared to those with CFLD (Smith et al, 2004). This suggests a possible protective role of UDCA against liver injury. Corbett et al reported that delayed diagnosis of CF predisposed to the development of CFLD. They also reported that children with established CFLD had evidence of impaired growth and nutrition and worse pulmonary status (Corbett et al, 2004). Several other studies have not found this association (Colombo et al, 2002; Slieker et al, 2003; Lamireau et al, 2004). The available data seem to indicate that nutritional status is not a causal factor of CFLD, but that patients with CFLD are at increased risk for deterioration of nutritional status (Corbett et al, 2004; Colombo et al, 2006).
A combination of factors causes secondary hepatocyte injury. Bile acid malabsorption results in retention of hepatotoxic glycine conjugated bile acids. Increased oxidative stress (Wood et al, 2001) and the release of inflammatory cytokines (Rosser & Gores, 1995) contribute to the development of progressive fibrosis, leading to focal biliary cirrhosis, the pathogonomic hepatic lesion in CF (Maurage et al, 1989). Focal biliary cirrhosis is the most clinically relevant hepatic problem associated with CF, since extension of the focal process may lead to multilobular biliary cirrhosis and the development of portal hypertension (Sokol et al, 1999). This process may take years or even decades during which there may be no clinical or biochemical manifestations of deteriorating liver health. As in other conditions characterised by initial involvement of bile ducts and later impairment of hepatocyte function, liver failure tends to be a late event (Colombo et al, 2004).
Incidence and prevalence
The true incidence of CFLD is difficult to determine as there are no sensitive or specific diagnostic markers. Earlier studies reported a peak incidence in adolescence, decreasing by the third decade of life (Scott-Jupp et al, 1991). However, in a later 15 year prospective study, 25% of children aged four years or older had biochemical markers of liver disease, with severe liver disease developing mainly during pre-puberty and puberty and a median age at diagnosis of 12 years (Lindblad et al, 1999). In the latter study no patient developed clinical liver disease in adulthood. During a 14 year median follow-up in a prospective assessment the incidence rate (number of new cases per year) of CFLD was 1.8% (Colombo et al, 2002). The mean age at diagnosis was 7.5 years with a sharp decline in incidence after the age of 10 years. In another prospective study the incidence of cirrhosis was 4.5% following a median period of five years from the diagnosis of CFLD (Colombo et al, 2002).
Prevalence (total number of cases) data for CFLD are similarly bedevilled by a lack of specific diagnostic markers and the different criteria used for patient selection in different studies. The reported prevalence ranges from 2% to 68% in children and adolescents, based upon whether the diagnosis is made on clinical, biochemical and/or ultrasound criteria. Early autopsy studies reported a progressive increase in the prevalence of focal biliary cirrhosis with age, from 10% in infants to 72% in adults (Vawter et al, 1979). The last decade has been characterised by the active search for CFLD and increased recognition of asymptomatic patients with focal biliary cirrhosis (Colombo et al, 2006). Studies in which CFLD has been actively searched for in large series of patients with CF, using biochemical and ultrasonographic assessment, have reported prevalence figures ranging from 18% to 37% (Colombo et al, 1994; Wilschanski et al, 1999). The most recent longitudinal study to assess the prevalence of CFLD in a large cohort of 241 patients showed presentation mainly in the first decade of life with a prevalence of 41% at 12 years of age and no increase thereafter (Lamireau et al, 2004). In the same study, the presence of CFLD was not associated with more severe pulmonary disease. The reported prevalence of portal hypertension varies from 1.7% to 8% (Sokol et al, 1999; Patriquin et al, 1999; Colombo et al, 2002; Lamireau et al, 2004).
Liver disease is thus a frequent and early complication of CF. Attempts at diagnosis should focus on the first decade of life.
Clinical presentation
Cystic fibrosis liver disease is often difficult to define clinically as it initially causes no symptoms and shows inconsistent abnormalities in serum liver function tests. Often abnormalities will only be seen on histology or ultrasound examination. The most common clinical presentation is an enlarged liver on routine physical examination, with or without associated abnormalities in liver biochemistry. Jaundice is generally limited to babies with neonatal cholestasis or to those with end-stage multilobular biliary cirrhosis. Although the overall incidence of CF as a cause of neonatal cholestasis is lower than 1% (Shapira et al, 1999), a sweat test should be part of the diagnostic work-up of cholestatic infants. Early detection can be difficult, but should be actively pursued because only early lesions are likely to be reversible.
Patients may present with complications of CFLD, the most severe of which is portal hypertension, suggested by splenomegaly, hypersplenism and upper gastrointestinal bleeding secondary to oesophageal or gastric varices (Colombo et al, 1999). As focal biliary cirrhosis progresses to multilobular cirrhosis the complications of portal hypertension appear at an unpredictable pace. As in other liver diseases characterised by initial involvement of the bile ducts, liver failure is a late event (Tanner et al, 1995). Once multilobular cirrhosis and portal hypertension are established the prognosis is poor. In one series 20% of patients died as a direct result of liver disease, with a mean survival of only 4.5 years (Noble-Jamieson et al, 1996).
Other hepatobiliary manifestations of CFLD
Steatosis
The most common hepatic lesion associated with CF is steatosis (fatty deposits in the liver), which can be detected in up to 67% of patients of any age. Its pathogenesis has not been definitely established, but it does not seem directly related to the CF secretory defect (Fields et al, 2006). Massive steatosis was once frequently observed in newly diagnosed patients with pancreatic insufficiency and severe malnutrition, but has now become less frequent due to earlier diagnosis and appropriate nutritional care. Fatty infiltration may be a result of the liver being involved as an “innocent bystander” (Colombo et al, 2004). In less severe forms, it has been associated with nutritional deficiencies (e.g. of essential fatty acids, carnitine, minerals and trace elements) and with antibiotic therapy. There is no evidence to suggest that steatosis progresses to cirrhosis and it is recognised as a relatively benign condition.
Biliary disease
Biliary manifestations of CFLD are seen in 5% to 33% of patients and include abnormalities of intra- and extrahepatic bile ducts, gallbladder thickening and contraction, microgallbladder and cholelithiasis (King et al, 2000). Gallstones seen in 12% to 24% of patients consist mostly of cholesterol stones. Microgallbladder was demonstrated in 25% of patients in an autopsy series. Intra- and extrahepatic biliary abnormalities are found in up to 50% of patients and vary from tapering of intrahepatic bile ducts to focal dilatation of larger bile ducts (Dietrich et al, 2002).
Screening for liver disease
Patients should be examined routinely for hepatosplenomegaly (large liver and spleen) at every visit. Upper abdominal ultrasound examination and serum liver function tests should be part of the annual assessment.
Liver function tests
Liver function test results may be intermittently elevated and do not always correlate with the severity of hepatic lesions. Twenty to 30% of children may have a single elevated liver enzyme at any given point in time (Diwakar et al, 2001). Transient elevation of liver function tests may be seen with hypoxaemia, antibiotic treatment and during a pulmonary exacerbation. Thus serial measurements should be performed. Liver enzymes originating from the biliary epithelium (gamma-GT, 5-nucleotidase and the biliary isoenzyme of alkaline phosphatase) are more specific for CFLD. Biochemical and ultrasonographic assessment may reflect different aspects of disease progression (Williams et al, 2002). A combination of several tests is needed to reliably detect liver disease. The United States CF Foundation recommends that significant liver disease should be suspected if any liver enzyme is more than 1.5 times the upper limit of normal on two occasions six months apart (Sokol et al, 1999).
Abdominal ultrasound
Upper abdominal ultrasound is the investigation of choice to look for hepatobiliary problems and for long term follow up (Williams et al, 1995; Stewart, 2005). As a screening tool it has advantages over other investigations. The relative paucity of body fat in children makes them good subjects. There is no ionising radiation. The procedure is usually well tolerated. Structures down to one millimetre size can be seen in detail and with very high resolution (Stewart, 2005). Liver size and texture, steatosis, the presence of associated splenomegaly and gallbladder abnormalities can be assessed.
Several studies have looked for a correlation between ultrasound findings and biochemical test results. In 195 children, 21% of those with normal ultrasound findings had abnormal biochemistry and 33% of those with an abnormal ultrasound had normal biochemistry (Patriquin et al, 1999). In a nine year review evaluating the ultrasound examination as part of the annual assessment 24% of cases revealed disparity between ultrasound findings and AST (a liver enzyme) levels (Williams et al, 2002). The ultrasound scan may thus select a subgroup of patients who have focal hepatic lesions with normal hepatic function. These patients are more likely to respond to early intervention therapy with ursodeoxycholic acid (UDCA).
For a better evaluation of portal hypertension, ultrasound can be used in association with Doppler studies of the portal vein, which can detect dilatation and flow patterns of the hepatic vasculature (Vergesslich et al, 1989). Portal hypertension is suggested by decreased portal venous flow velocities or reversal of flow (hepatofugal) in the portal vein.
Scintigraphy
Hepatobiliary scintigraphy with the technetium labelled isotope DISIDA represents another non-invasive means of assessing both liver function and biliary excretion. The DISIDA scan has a role as a complementary investigation to ultrasound examination (O’Connor et al, 1996). The most common abnormality seen is beading of the intrahepatic ducts, followed by retention of activity within the liver, increased visualisation of secondary ducts and non-visualisation of the gallbladder. Fourteen of the 44 patients in O’Connor’s study were re-examined after a mean interval of 4.7 years (Foster et al, 2002). Results from this study showed that a combination of investigations is required for reliable follow-up of liver disease. This is probably because different parameters of liver status are measured by the different tests. Delayed intestinal visualisation at hepatobiliary scintigraphy is associated with a better response to long term treatment with UDCA (Colombo et al, 1999).
Computed tomography and magnetic resonance imaging
Several studies have described magnetic resonance (MR) and computed tomography (CT) findings in patients with cystic fibrosis (King et al, 2000; Akata et al, 2002). Magnetic resonance cholangiography (MRC) is a non-invasive procedure that provides a high quality picture of any abnormalities of the intra- and extra-hepatic bile ducts (King et al, 2000). Patients with CFLD show abnormal findings on MRC. Repeat MR examinations can be performed to evaluate disease progression or response to therapy without any radiation exposure, or the difficulties encountered by comparison of findings in serial ultrasound examinations performed by different observers (Akata et al, 2007).
Liver biopsy
The role of percutaneous liver biopsy is controversial. It is an invasive procedure and early changes of liver disease are focal in nature and may therefore be missed by blind biopsy. Even though significant liver disease can be detected by biopsy in the presence of normal clinical and ultrasound examinations and normal liver enzyme levels (Davidson et al, 2000), a normal biopsy cannot exclude even advanced liver disease (Lindblad et al, 1999). Biopsy is associated with significant morbidity (3%) and mortality (0.03%). Nevertheless, liver biopsy may provide important information on the type of the predominant lesion (steatosis or focal biliary cirrhosis), the extent of portal fibrosis, rate of progression and response to treatment. It should be performed if there is doubt about the diagnosis and to establish cirrhosis prior to treatment or transplantation (Colombo et al, 2006).
Treatment of liver disease
Medical treatment
Therapy is aimed at improving biliary excretion and bile acid composition. UDCA is a naturally occurring hydrophilic bile acid which improves biochemical indices of liver function (Colombo et al, 1990; Sokol et al, 1999). Ursodeoxycholic acid augments bile flow, displaces toxic hydrophobic bile acids, stimulates bicarbonate secretion into bile and has a general cytoprotective cholangiocyte effect (Kowdley, 2000; Gores, 2000). A 10 year prospective study of the effect of UDCA showed promising results in its ability to arrest the progression of early focal lesions (Nousia-Arvanitakis et al, 2001). Seventy individuals with ultrasound evidence of multifocal liver disease were commenced on UDCA. After 10 years the progression of nodular biliary cirrhosis, shown by ultrasound examination, was arrested, hepatic function preserved and no variceal bleeding observed. No case of focal biliary cirrhosis progressed to nodular biliary cirrhosis, and ultrasound changes suggestive of focal biliary cirrhosis were reversed. Biochemical indices returned to normal levels within a year and remained normal to 10 years follow-up.
Ursodeoxycholic acid is commenced at 20 mg/kg/day in two or three divided doses when early abnormalities on ultrasound are seen and/or persistent raised liver function tests are documented. Its effect is dose dependent and a high dose is necessary to compensate for poor absorption. Although a randomised, double-blind trial showed no significant effect of taurine supplementation on liver function test results (Colombo et al, 1996), taurine deficiency is common in patients with CF as a result of bile acid malabsorption (Weber & Roy, 1985; Thompson, 1988). Long term administration of unconjugated UDCA may critically increase the demand for taurine needed for bile acid conjunction, and depletion of taurine induced by large doses of exogenous bile acid has been demonstrated in patients with gallstones (Batta et al, 1982). Patients given taurine also show a significant improvement in serum prealbumin levels, a sensitive indicator of nutritional status and a trend to less fat malabsorption (Colombo et al, 1996). Thus our patients are supplemented with taurine at a dose of 30 mg/kg/day, in two or three divided doses.
Information is available on the effect of UDCA treatment in CFLD from several randomised controlled trials. All studies show a consistent and sustained improvement in biochemical indices (Colombo et al, 1996). Improvements in hepatic excretory function and biliary composition have been documented. Only preliminary data on its effects on liver histology are available. In a limited number of patients a marginal improvement was seen in inflammation and fibrosis following two years of treatment with UDCA (Lindblad et al, 1998). In another study, eight of 10 patients were rebiopsied after one year of treatment with UDCA and showed histological improvement (Wong et al, 1997).
Direct evidence that UDCA can prevent or reverse progressive fibrosis is, however, not available. Use of UDCA is widespread, even though the Cochrane review in 2000 concluded that there was insufficient evidence to justify its routine use (Cheng et al, 2000).
Taking all the above results into consideration we believe that it is essential to commence patients on UDCA at an early stage since only the early lesions are likely to be reversible.
Treatment of portal hypertension
Variceal bleeding is a life-threatening complication of portal hypertension and may precipitate hepatocellular failure. Management should concentrate on the control of any bleeding and on variceal decompression. In one study, 50% of children who developed oesophageal varices bled early in their second decade (Debray et al, 1999). The preferred initial intervention for bleeding varices is upper gastrointestinal endoscopy with injection sclerotherapy or band ligation (Stringer et al, 1993; Price et al, 1996; Debray et al, 1999; Efrati et al, 2003; Brigman & Feranchak, 2006). Injection sclerotherapy has an 86% success rate without significant morbidity (Stringer et al, 1993). Prophylactic sclerotherapy is not beneficial in CF (D’Amico et al, 1995). In older children and adults band ligation has replaced sclerotherapy as first line treatment in secondary prevention of variceal haemorrhage (Brigman & Feranchak, 2006). Elastic bands are placed around varices using a device attached to the end of the endoscope. Mechanical compression and thrombosis occurs which cause eradication of the varix. The efficacy of beta-blockers has not been evaluated in CF because of their potential adverse effects on airway reactivity.
Surgical portosystemic shunting can permanently reduce portal pressure and relieve portal hypertension in patients without progressive liver and lung failure (Debray et al, 1999; Efrati et al, 2003). Specific indications include large varices at high risk of bleeding, bleeding from gastric varices and failure of endoscopic control of bleeding. Potential complications of portosystemic shunting include onset or worsening of hepatic encephalopathy, shunt thrombosis or occlusion. Operative shunts may also make eventual liver transplantation more difficult.
Non-surgical shunting with the placement of a transjugular intrahepatic portosystemic shunt (TIPSS) can be used for patients with variceal haemorrhage refractory to sclerotherapy. The shunt consists of a fenestrated metal stent placed between the hepatic and portal vein via the jugular vein. It can also be performed on an emergency basis in patients who are actively bleeding, or in those who exhibit rapid progression to liver failure (Debray et al, 1999). Transjugular intrahepatic portosystemic shunt can effectively control variceal bleeding, both as a long-term therapy for portal hypertension and as a bridge for liver transplantation (Brigman & Feranchak, 2006). Transjugular intrahepatic portosystemic shunt controls active variceal bleeding in more than 90% of cases. Complications include TIPSS dysfunction with a stenosis, occlusion or thrombosis and new-onset or worsened encephalopathy. Shunt stenosis is the principal limiting factor of TIPSS, but it can be easily diagnosed with sonography, and dilated with balloon angioplasty before the clinical signs of portal hypertension occur. Regular sonographic examinations are a necessity (Pozler et al, 2003). The technique and complications in children are comparable to those in adults. Case reports make up the current literature for TIPSS in children with CFLD (Fleet et al, 2000; Pozler et al, 2003). In the latter study, TIPSS was successfully performed in five children with a median follow-up of 70 months, but multiple re-interventions were required for shunt stenosis. In one patient, TIPSS served as a bridge to liver transplantation.
Treatment of splenomegaly
Massive splenomegaly can cause significant abdominal discomfort, gastric compression leading to impaired nutritional intake and impaired diaphragmatic function causing dyspnoea. Different techniques to reduce the spleen volume have been attempted including partial or total splenectomy and partial splenic embolisation.
Experience with total splenectomy for massive splenomegaly in CF is limited. Patients may benefit from splenectomy at a time when their lung disease is stable and thus anaesthetic risks are low (Taylor & Ward, 2000). Concern remains over fulminant, potentially life-threatening infection from loss of splenic macrophages, and the procedure carries a mortality of up to 15%. A case series of nine patients with CF had total splenectomy performed at a mean age of 14.8yrs (Linnane et al, 2006). The three most common indications were low platelet count, risk of splenic rupture and hypersplenism. The mean FEV1 % predicted declined by 8% per year in the two years prior to splenectomy; approximately four times the rate for the CF population as a whole. This accelerated decline halted post-splenectomy. No patient developed overwhelming sepsis during a median follow-up of six years. These findings are consistent with previous publications which show no single absolute indication for splenectomy. Instead, a combination of factors tips the risk:benefit ratio in favour of operative intervention. In the most recent case series (Robberecht et al, 2006) six patients underwent successful surgery with a median follow-up of 61 months. All patients had massive splenomegaly and variceal bleeding as indications for splenectomy. One patient died of overwhelming septicaemia. Immuno- and chemoprophylaxis is indispensable following splenectomy to minimize septic complications, and some centres only perform the procedure in children over the age of 10 years (Robberecht et al, 2006).
Partial splenectomy with conservation of the upper pole of the spleen may be the preferential treatment in patients with CF, but experience is limited (Thalhammer et al, 2003). After partial splenectomy, splanchnic blood flow is decreased, resulting in reduced portal pressure and a diminution of oesophageal varices. Up to 95% of the spleen can be safely removed, decreasing the spleen’s volume and blood supply, while maintaining its immunological functions. Chazalette et al reported a successful outcome in 11 patients. Improvement of oesophageal varices was noted in nine cases and all patients observed had a speedy normalisation of their haematological profile (Chazalette et al, 2003). A further advantage of partial splenectomy is that it allows a delay of liver transplantation which may even be avoided altogether.
The debate on the justification for removing all or part of the spleen in patients with CF continues (Westwood et al, 2004). Hypersplenism in the absence of significant consequences is not on its own an indication for this major procedure. Quality of life and the local effects of the size of the spleen may, however, justify the surgical and immunological risk.
Partial splenic embolisation (PSE) has been reported in a limited number of patients with CF (Brandt et al, 1989; Shah et al, 1990; Aslanidou et al, 2007). It decreases splenic blood flow reducing portal pressure, and improves peripheral cytopenia. The procedure was initially associated with significant morbidity and mortality and a high complication rate. Subsequent series reported substantially decreased complications, and it now has an acceptably low incidence of serious complications, with mortality not exceeding 6% (N’Kontchou et al, 2005). In patients with CF it can provide long lasting efficacy in controlling hypersplenism, with duration of follow-up now more than 14 years (Aslanidou et al, 2007). Paediatric angiographic expertise is essential. A painful postembolisation period may cause impaired respiratory movements of the left lung needing extra physiotherapy input.
Key points
• CFLD is the third most common cause of death in people with CF
• Onset of CFLD is usually in early childhood
• The true incidence and prevalence of CFLD is difficult to assess because there is no single, reliable diagnostic test
• The most common clinical presentation is hepatomegaly
• Clinical examination for hepatomegaly should be performed regularly
• Upper abdominal ultrasound and blood liver function tests should form part of the annual assessment
• Treatment with UDCA should begin early when abnormalities are detected clinically, on routine blood tests, or on ultrasound examination
References
Akata D, Akhan O, Ozcelik U, et al. Hepatobiliary manifestations of cystic fibrosis in children: correlation of CT and US findings. Eur J Radiol 2002, 41: 26-33. [PubMed]
Akata D, Akhan O. Liver manifestations of cystic fibrosis. Eur J Radiol 2007; 61: 11-17. [PubMed]
Aslanidou E, Fotoulaki M, Tsitouridis I, et al. Partial splenic embolisation: successful treatment of hypersplenism, secondary to biliary cirrhosis and portal hypertension in cystic fibrosis. J Cyst Fibros 2007; 6: 212-214. [PubMed]
Batta AK, Salen G, Shefer S, et al. The effect of tauroursodeoxycholic acid and taurine supplementation on biliary bile acid composition. Hepatology 1982; 2: 811-816. [PubMed]
Brigman C, Feranchak A. Liver involvement in cystic fibrosis. Curr Treat Options Gastroenterol 2006; 9: 484-496. [PubMed]
Brandt CT, Rothbart LJ, Kumpe D, et al. Splenic embolisation in children: long term efficacy. J Pediatr Surg 1989; 24: 642-645. [PubMed]
Castaldo G, Fuccio A, Salvatore D, et al. Liver expression in cystic fibrosis could be modulated by genetic factors different from the cystic fibrosis transmembrane regulator genotype. Am J Med Genet 2001; 98: 294-297. [PubMed]
Chazalette JP, Fiegelson J, Louis D. Partial splenectomy – worth the risk? Arch Dis Child 2003; 88: 649. [PubMed]
Cheng K, Ashby D, Smyth RL. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst Rev 2000; 2: CD000222. [PubMed]
Collardeau-Frachon S, Bouvier R, Le Gall C, et al. Unexpected diagnosis of cystic fibrosis at liver biopsy: a report of four pediatric cases. Virchows Arch. 2007 Jul;451(1):57-64. [PubMed]
Colombo C, Setchell KD, Podda M, et al. Effects of ursodeoxycholic acid therapy for liver disease associated with cystic fibrosis. J Pediatr 1990; 117: 482-489. [PubMed]
Colombo C, Apostolo MG, Ferrari M, et al. Analysis of risk factors for the development of liver disease in CF. J Pediatr 1994; 124: 393-399. [PubMed]
Colombo C, Battezatti PM, Podda M. Hepatobiliary disease in cystic fibrosis. Semin Liv Dis 1994; 14: 259-269. [PubMed]
Colombo C, Battezatti PM, Podda M, et al. Ursodeoxycholic acid for liver disease associated with cystic fibrosis: a double blind multicentre trial. Hepatology 1996; 23: 1484-1490. [PubMed]
Colombo C, Crosignani A, Battezzati PM. Liver involvement in cystic fibrosis. J Hepatol 1999; 31: 946-954. [PubMed]
Colombo C, Crosignani A, Battezzati PM, et al. Delayed intestinal visualization at hepatobiliary scintigraphy is associated with response to long-term treatment with ursodeoxycholic acid in patients with cystic fibrosis-associated liver disease. J Hepatol 1999; 31: 672-677. [PubMed]
Colombo C, Battezzati PM, Crosignani A, et al. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors and outcome. Hepatology 2002; 36: 1374-1382. [PubMed]
Colombo C, Battezzati PM. Liver involvement in cystic fibrosis: primary organ damage or innocent bystander? J Hepatol 2004; 41: 1041-1044. [PubMed]
Colombo C, Russo MC, Zazzeron L, et al. Liver disease in cystic fibrosis. J Paed Gastroenterol Nutr 2006; 43: S49-S55. [PubMed]
Cohn JA, Strong TV, Picciotto MR, et al. Localisation of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology 1993; 105:1857-1864. [PubMed]
Corbett K, Kelleher S, Rowland M, et al. Cystic fibrosis-associated liver disease: a population based study. J Pediatr 2004; 145: 327-332. [PubMed]
Cystic Fibrosis Foundation (CFF). CFF Patient Registry Annual Data Report. Bethesda, Maryland, 2002.
D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22: 332-354. [PubMed]
Davidson AGF, Jamieson D, Jevon Y, et al. Detection of liver disease in CF patients: results from clinical evaluation, liver enzymes, ultrasound and biopsy. Abstract book, XIIIth International CF Congress, Stockholm, 2000:132.
Debray D, Lykavieris P, Gauthier F, et al. Outcome of cystic fibrosis associated liver cirrhosis: management of portal hypertension. J Hepatol 1999; 31: 77-83. [PubMed]
Dietrich C, Chichakli M, Hirche T, et al. Sonographic findings of hepatobiliary-pancreatic system in adult patients with cystic fibrosis. J Ultrasound Med 2002; 21: 409-416. [PubMed]
Diwakar V, Pearson L, Beath S. Liver disease in children with cystic fibrosis. Paediatr Respir Rev 2001; 2: 340-349. [PubMed]
Efrati O, Barak A, Modan-Moses D, et al. Liver cirrhosis and portal hypertension in cystic fibrosis. Eur J Gastroenterol Hepatol 2003; 15: 1073-1078. [PubMed]
Fields TM, Michael SJ, Butler CL, et al. Abdominal manifestations of cystic fibrosis in older children and adults. Am J Roentgenol 2006; 187: 1199-1203. [PubMed]
Fleet M, Stanley AJ, Forrest EH, et al. Transjugular intrahepatic portosystemic shunt placement in a patient with cystic fibrosis complicated by portal hypertension. Clin Radiol 2000; 55: 236-237. [PubMed]
Foster JA, Ramsden WH, Conway SP, et al. The role of IDA scintigraphy in the follow-up of liver disease in patients with cystic fibrosis. Nucl Med Commun 2002; 23: 673-681. [PubMed]
Friedman KJ, Ling S, Handler A, et al. High throughput SNP genotyping as a strategy to identify additional genetic modifiers in the complex multigenic mechanisms underlying severe CF liver disease. Pediatr Pulmonol 2002; Suppl 24: 223.
Gores G. Mechanisms of cell injury and death in cholestasis and hepatoprotection by ursodeoxycholic acid. J Hepatol 2000; 32: S11-S13.
Henrion-Caude A, Flamant C, Roussen M, et al. Liver disease in CF patients is associated with glutathione S-transferase P1 polymorphism. Hepatology 2002; 36: 913-917. [PubMed]
King L, Scurr E, Murugan N, et al. Hepatobiliary and pancreatic manifestations of cystic fibrosis: MR imaging appearances. Radiographics 2000, 20: 767-777. [PubMed]
Kinnman N, Lindblad A, Housset C, et al. Expression of cystic fibrosis transmembrane conductance regulator in liver tissue from patients with cystic fibrosis. Hepatology. 2000; 32: 334-340. [PubMed]
Kowdley K. Ursodeoxycholic acid therapy in hepatobiliary disease. Am J Med 2000; 108: 481-486. [PubMed]
Lamireau T, Monnereau S, Martin S, et al. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41: 920-925. [PubMed]
Lewindon PJ, Pereira TN, Hoskins AC, et al. The role of hepatic stellate cells and transforming growth factor-beta (1) in cystic fibrosis. Am J Pathol 2002; 160: 1705-1715. [PubMed]
Lindblad A, Glaumann H, Strandvik B. A two year prospective study of the effect of ursodeoxycholic acid on urinary bile excretion and liver morphology in cystic fibrosis associated liver disease. Hepatology 1998; 27: 166-174. [PubMed]
Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology 1999; 30: 1151-1158. [PubMed]
Linnane B, Oliver MR, Robinson PJ. Does splenectomy in cystic fibrosis related liver disease improve lung function and nutritional status? A case series. Arch Dis Child 2006; 91: 771-773. [PubMed]
Maurage C. Lenaerts C, Weber AM, et al. Meconium ileus and its equivalent as a risk factor for the development of cirrhosis: an autopsy study in cystic fibrosis. J Paediatr Gastroenterol Nutr 1989; 9: 17-20. [PubMed]
N’Kontchou G, Seror O, Bourcier V, et al. Partial splenic embolisation in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol 2005; 17: 179-184. [PubMed]
Noble Jamieson G, Barnes N, Jamieson N, et al. Liver transplantation for hepatic cirrhosis in cystic fibrosis. J R Soc Med 1996; 89(Suppl 27): 31-37. [PubMed]
Noble-Jamieson G, Barnes ND. Liver transplantation for cirrhosis in cystic fibrosis. J Pediatr 1996; 129: 314. [PubMed]
Nouisa-Arvanitakis S, Fotoulaki M, Economou H, et al. Long term prospective study of the effect of ursodeoxycholic acid on cystic fibrosis related liver disease. J Clin Gastroenterol 2001; 32: 324-328. [PubMed]
O’Connor PJ, Southern KW, Bowler IM, et al. The role of hepatobiliary scintigraphy in cystic fibrosis. Hepatology 1996; 23: 281-287. [PubMed]
Patriquin H, Lenaerts C, Smith L, et al. Liver disease in children with CF: US-biochemical comparison in 195 patients. Radiology 1999; 211: 229-232. [PubMed]
Pozler O, Krajina MD, Vanicek H, et al. Transjugular intrahepatic portosystemic shunt in five children with cystic fibrosis: long term results. Hepatogastroenterology 2003; 50: 1111-1114. [PubMed]
Price MR, Sartorelli KH, Karrer FM, et al. Management of oesophageal varices in children by endoscopic variceal ligation. J Pediatr Surg 1996; 31: 1056-1059. [PubMed]
Rosser BG, Gores GJ. Liver cell necrosis: Cellular mechanisms and clinical implications. Gastroenterology 1995; 108: 252-275. [PubMed]
Robberecht E, van Biervliet S, Vanrentergem K, et al. Outcome of total splenectomy with portosystemic shunt for massive splenomegaly and variceal bleeding in cystic fibrosis. J Pediatr Surg 2006; 41: 1561-1565. [PubMed]
Salvatore F, Scudiero O, Castaldo G. Genotype-phenotype correlation in cystic fibrosis: the role of modifier genes. Am J Med Genet 2002; 111: 88-95. [PubMed]
Scott-Jupp R, Loma M, Tanner MS. Prevalence of liver disease in cystic fibrosis. Arch Dis Child 1991; 66: 698-701. [PubMed]
Shah R, Mahour GH, Ford EG, et al. Partial splenic embolization. An effective alternative to splenectomy for hypersplenism. Am Surg. 1990; 56: 774-777. [PubMed]
Shapira R, Hadzic W, Francavilla R, et al. Retrospective review of cystic fibrosis presenting as infantile liver disease. Arch Dis Child 1999; 81: 125-128. [PubMed]
Slieker MG, Deckers-Kocken JM, Vitterwaal CSPM, et al. Risk factors for the development of cystic fibrosis-related liver disease. Hepatology 2003; 38: 775-776. [PubMed]
Smith JL, Lewindon PJ, Hoskins AC, et al. Endogenous ursodeoxycholic acid and cholic acid in liver disease due to cystic fibrosis. Hepatology 2004; 39: 1673-1682. [PubMed]
Sokol RJ, Durie PR. Recommendations for management of liver and biliary tract disease in cystic fibrosis. J Pediatr Gastroenterol Nutr 1999; 28: S1-13. [PubMed]
Stewart L. The role of abdominal ultrasound in the diagnosis, staging and management of cystic fibrosis liver disease. J R Soc Med 2005; 98(Suppl 45): 17-27.
Stringer MD, Price JF, Mowat AP, et al. Liver cirrhosis in cystic fibrosis. Arch Dis Child 1993; 69: 407. [PubMed]
Tanner MS, Taylor CJ. Liver disease in cystic fibrosis. Arch Dis Child 1995; 72: 281-284. [PubMed]
Thalhammer GH, Eber E, Uranus S, et al. Partial splenectomy in cystic fibrosis patients with hypersplenism. Arch Dis Child 2003; 88: 143-146. [PubMed]
Taylor CJ, Ward C. Long term outcome of splenectomy in CF liver disease. Abstract book, XIIIth International CF Congress, Stockholm, 2000: 132.
Thompson GN. Excessive fecal taurine loss predisposes to taurine deficiency in cystic fibrosis. J Pediatr Gastroenterol Nutr 1988; 7: 214-217. [PubMed]
Vawter GF, Schwachman H. Cystic fibrosis in adults: an autopsy study. Pathology Annual 1979; 14: 357-382. [PubMed]
Vergesslich KA, Gotz M, Mostbeck G, et al. Portal veins blood flow in cystic fibrosis: assessment by duplex Doppler sonography. Pediatr Radiol 1989; 19: 371-374. [PubMed]
Weber AM, Roy CC. Bile acid metabolism in children with cystic fibrosis. Acta Paediatr Scand 1985; 317(Suppl): 9-15. [PubMed]
Westwood ATR, Millar AJW, Ireland JD, et al. Splenectomy in cystic fibrosis patients. Arch Dis Child 2004; 89: 1078. [PubMed]
Wilschanski M, Rivlin J, Cohen S, et al. Clinical and genetic risk factors for cystic fibrosis related liver disease. Pediatrics 1999; 103: 52-57. [PubMed]
Williams SG, Evanson JE, Barrett N, et al. An ultrasound scoring system for the diagnosis of liver disease in cystic fibrosis. J Hepatol 1995; 22: 513-521. [PubMed]
Williams SM, Goodman R, Thomson A, et al. Ultrasound evaluation of liver disease as part of an annual assessment clinic: a 9 year review. Clin Radiol 2002; 57: 365-370. [PubMed]
Wong LTK, Davidson AGF, Jevon G, et al. Hepato-biliary disease in cystic fibrosis: enzymatic and histopathologic response to ursodeoxycholic acid therapy. Pediatr Pulmonol 1997; Suppl 14: 303.
Wood LG, Fitzgerald DA, Gibson PG, et al. Oxidative stress in Cystic Fibrosis: Dietary and metabolic factors. J Am Coll Nutr 2001; 20: 157-165. [PubMed]
