Low bone mineral density and osteoporosis in CF
Introduction
People with CF can now look forward to adult life as long as they receive optimal care from a CF Centre. However, as the natural history of CF in adults unfolds both carers and patients must deal with clinical issues that were not problems when it was a disease only of childhood. Thin bones are one such problem. Rheumatic disorders with joint pain are another.
The normal cycle of bone regeneration
Throughout life the skeleton is constantly remodelled by a co-ordinated programme of cellular activity that removes, renews and repairs damaged bone. About 10% of the adult skeleton is remodelled each year. Specialised cells, perhaps stimulated by factors released when bone undergoes even microscopic damage, resorb a specific amount of bone from the damaged areas before undergoing a programmed cell death. The repair process is then continued by further specialised cells that form the new bone.
Osteoporosis
Osteoporosis is a complicated disease of the skeleton resulting in a deficiency of bone mass and a reduced bone density. As a consequence the bones’ architectural structure is altered so that it is more fragile and more likely to fracture. Peak bone mass is achieved in early adulthood and bone mineral density (BMD) will not increase thereafter. On the contrary, after a period of several years stability it will begin to decrease at around thirty-five years of age, and given the wrong combination of environmental (smoking, poor diet, large alcohol intake, little weight bearing exercises) and genetic influences this will almost inevitably result in osteoporosis in old age.
Measurement of BMD
Bone mineral density is most easily measured by a technique called dual energy X-ray absorptiometry (DXA) scanning. This is an entirely painless scan available in most large hospitals. Bone mineral density results for any individual can be compared to the mean BMD of a population of young adults (a T-score), or to the mean value for the patient’s age and gender matched controls obtained from DXA studies of the general population (a Z-score). Z-scores should always be used for children who are yet to reach their peak bone mass (Specker & Schoenau, 2005). Reference databases should accurately reflect gender and ethnicity (Gafni & Baron, 2004). In people with CF low BMD seems to first appear in early adolescence (Greer et al, 2003; Buntain et al, 2004; Bianchi et al, 2006). We believe therefore that patients over ten years of age should be screened by DXA scanning. Scans should be repeated every one to three years or as determined by clinical need to ensure satisfactory bone mineral accrual (Cystic Fibrosis Trust, 2007).
Definitions
Osteoporosis or osteopenia are diagnosed on World Health Organisation (WHO) criteria when the BMD T-score falls more than 2.5 or between 1.0 and 2.5 standard deviations respectively below the young normal adult mean. Results should be interpreted with caution in people with CF, allowing for the fact that the two-dimensional DXA measurement will underestimate BMD in short, narrow bones (Wren et al, 2005). Because the relationship between the WHO definition of osteoporosis and fracture risk has only been validated for post menopausal women, the CF Trust advises that the term osteoporosis should only describe those patients with CF who have a history of fragility fracture. Other patients with a BMD Z-score below -2 should be described as having CF related low BMD (Cystic Fibrosis Trust, 2007).
Low BMD in CF
Reduced BMD in patients with CF was first documented in 1979 (Mischler et al, 1979). Subsequently researchers have found reduced bone mineral content common at all ages in people with CF, suggesting the importance of decreased accretion of bone mineral content as well as increased loss (Bhudhikanok et al, 1998; Buntain et al, 2006). Low BMD is common in adult patients (Haworth et al, 1999; Conway et al, 2000). The pattern of complications from low BMD in older patients with CF is similar to that in the non-CF population but seen about thirty years earlier. Low BMD shows a definite relationship with disease severity being common in patients on the transplant list and following transplant surgery (Aris et al, 1998; Donovan et al, 1998). About 75% of these patients show severely reduced bone mineral content. This certainly reflects in part the associated post-operative inactivity and high dose corticosteroid treatment given for rejection episodes.
Aetiology of low BMD in CF
If fine control of the resorption/renewal process is disturbed bones may become pathologically ‘thin’ i.e. osteoporotic. Studies in CF have shown either a failure of new bone formation, an increase in bone resorption, or both (Haworth et al, 2000; Aris et al, 2002; Elkin et al 2002; Greer et al, 2003). The aetiology of low BMD in CF is almost certainly a complex interaction of multiple factors including the effect of the CFTR mutation itself, poor nutrition, recurrent chest infections with raised levels of circulating pro-inflammatory cytokines, delayed puberty, hypogonadism, diabetes mellitus, reduced weight bearing exercise and treatment with corticosteroids.
Fracture risk with low BMD
There is no defined association between paediatric BMD values and fracture risk (Wren et al, 2005). Mild to moderate CF is not associated with an increased fracture risk in children and adolescents (Rovner et al, 2005). However, experience in the non-CF population is that thin bones do fracture more easily. The results of a recent meta-analysis suggest an association between low BMD and fractures in children. The authors recommend a large prospective cohort study to confirm their tentative conclusions (Clark et al, 2006). Fracture rates twice that in the general population were reported in young adults with CF (Aris et al, 1998).
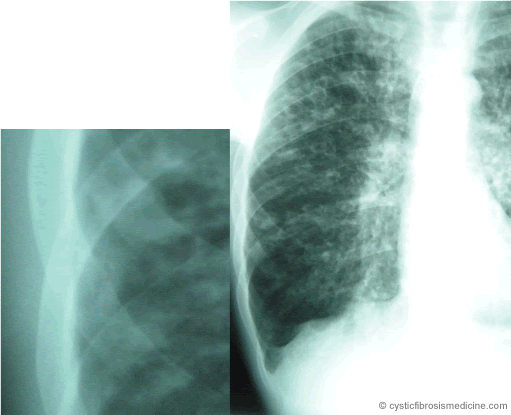
Osteoporotic bones in the spine are susceptible to ‘compression’ fracture so that they collapse down between the bones above and below. This can be very painful, especially if nerves are trapped within the resulting disorganised structure. The usually straight backbone can become abnormally curved. In severe spinal osteoporosis height loss can be as much as two to three inches. Structural changes in the chest wall can interfere with patients’ breathing patterns. Fractures occurring even in the absence of accidental injury may complicate the postoperative course after lung transplant and impair patients’ quality of life through resulting debility and pain.
Prevention of low BMD
Prevention of osteoporosis is clearly preferable to treatment and must be addressed. Glucocorticoids should be prescribed with caution. They initially stimulate bone resorption and with prolonged use suppress bone formation (Gluck & Colice, 2004). We have documented an association between reduced BMD and corticosteroid use (Conway et al, 2000).
Adequate nutrition is of the utmost importance. The natural ageing process will place any nutritionally deficient person at risk of osteoporosis whilst still a relatively young adult. Calcium is the major mineral in the skeleton. Reduced calcium intake in childhood is linked with an increased risk of osteoporosis in adult life and an increased calcium intake may improve BMD (Cadogan et al, 1997). Even though the efficiency of calcium absorption is not compromised in clinically stable CF patients (Schulze et al, 2003), girls with CF have significant endogenous faecal calcium loss (Schulze et al, 2003), and lower bone calcium accretion rates during pre- and late puberty than girls without CF (Schulze et al, 2006). Dietetic supervision is essential to ensure an adequate calcium intake (as well as for general nutritional monitoring).
Similarly vitamin D intake and blood levels should be reviewed at least annually. Low blood vitamin D levels are common in CF despite daily supplementation with at least 800 IU (Pike et al, 2000). We have revised our lower limit of normal, now aiming for a minimum blood vitamin D level of 30 ng/ml (75 nmol/L), (Bischoff-Ferrari et al, 2006). The vitamin D supplement is routinely given with vitamin A in a combined capsule. Where blood vitamin A levels are satisfactory but vitamin D levels low we prescribe additional vitamin D with calcium (Calceos®), usually one tablet per day in children and two per day in adults. Ergocalciferol, 250 µg/day is used with caution in recalcitrant cases. The major contribution to total body vitamin D, however, is the conversion of 7-dehydrocholesterol to colecalciferol in the skin through sunlight exposure. Just a few minutes daily sunlight exposure is needed. In our paediatric clinic the mean blood vitamin D level was 9.7 ng/ml in winter and 21.5 ng/ml in summer (Wolfe et al, 2001). Since we have increased our efforts to maintain a higher and seasonally consistent vitamin D level of around 30 ng/ml we have largely abolished this winter trough. N.B. a normal summer vitamin D level does not guarantee that levels are adequate for the rest of the year.
Increased attention is now focused on the role of vitamin K in bone health (Weber, 2001; Nicolaidou et al, 2006). Vitamin K is a fat soluble vitamin like vitamins A, D and E. Many patients with CF are therefore vitamin K deficient (Rashid et al, 1999; Conway et al, 2005). Osteocalcin plays an important role in bone formation and to function effectively needs to be converted by a vitamin K dependent process from its uncarboxylated to its carboxylated form. Better vitamin K status has been associated with decreased bone turnover in healthy non-CF girls (Kalkwarf et al, 2004). We have shown that vitamin K deficiency in children with CF is associated with increased bone turnover but could not show any direct correlation between the levels of carboxylated and undercarboxylated osteocalcin and BMD status. Vitamins K and D together may postpone fracture risk by 10 years in the non-CF population (Vermeer, 2004). Evidence is accumulating that all patients with CF should receive vitamin K supplements. The CF Trust recommends 300µg/kg/day for babies, 5mg/day for children aged two to seven years, and 10mg/day for older children and adults as Vitamin K1, phytomenadione (Cystic Fibrosis Trust, 2007).
Exercise should be actively encouraged not only for the way it complements chest physiotherapy, but because weight bearing activity increases bone mineral content (Janz et al, 2006). Smoking, of course, and excess alcohol should be avoided.
Treatment of low BMD
Specific drug therapy is available for osteoporosis but should only be considered when risk factors other than CF itself have been excluded, e.g. hypogonadism, abnormal thyroid function. Treatment with testosterone should be considered in young men over 16 years of age with reduced BMD and pubertal delay. We recommend that such treatment is only given in consultation with an endocrine specialist. Similarly, post-menopausal women with CF should be advised about the appropriateness of hormone replacement therapy by an endocrine specialist. This treatment may retard bone loss.
Bisphosphonates are effective treatments for low BMD, predominantly decreasing bone resorption but also having a positive effect on bone formation. They have been used successfully in CF (Aris et al, 2000; Haworth et al, 2001; Aris et al, 2004; Conway et al, 2004). Examples include alendronic acid and risedronate (oral preparations) and pamidronate and zolendronic acid (intravenous preparations). Potential problems with bisphosphonate drugs are –
Absorption from the gut is poor and may be further reduced in people with CF. They should be taken on an empty stomach.
Some of them, e.g. alendronate, can cause oesophageal ulceration and should be taken standing up and with a glass of water, and without lying down for half an hour to ensure rapid passage down the oesophagus and into the stomach.
The above administration problems may be reduced by prescribing the newer once weekly preparations. Almost all of our patients in Leeds, however, have tolerated these drugs without any problems.
Intravenous pamidronate is administered every three months but may cause bone pain in the days following the infusion (Haworth et al, 2001). This was not seen in patients receiving regular corticosteroids following lung transplant (Aris et al, 2000). We have found that oral prednisolone 20 mg as a single daily dose, given on the day before, the day of, and the day after the infusion prevents bone pain in most patients. Tolerance to pamidronate tends to occur with repeated infusions allowing gradual reduction and withdrawal of prophylactic glucocorticoids. Alternatively non-steroidal anti-inflammatory drugs or paracetamol may be tried (Robinson et al, 2004).
Bisphosphonates have been associated with a rare condition affecting the jaw bone called osteonecrosis of the jaw (Marx et al, 2005). Infection causes bone death and can be very difficult to successfully treat. This complication is mostly seen in patients treated for osteoporosis resulting from cancers or their treatments and in patients with poor dental hygiene. The risk is about one in every 60,000 patients treated with bisphosphonates. We recommend that each patient is informed of this very rare but potential possibility (to the best of our knowledge it has not been reported in any patient with CF), that treatment continues, that patients make sure they have six monthly dental checks and that they inform their dentists that they are taking a bisphosphonate.
It is important that these issues are discussed with patients. We recommend that bisphosphonates should only be prescribed in CF Centres.
The pain associated with rib fractures can often be very difficult to control with standard analgesia (NSAID and opiates). An alternative therapy is calcitonin (Jones et al, 2001). They found calcitonin to be highly effective in one of their patients with persistent pain despite standard analgesia. We have used similar treatment to good effect in a small number of patients.
Prevention is the key to osteoporosis treatment but some patients with collapsed vertebrae can have some of their pain relieved by injecting an acrylic resin into the collapsed vertebra so strengthening it from the inside.
The future
Today’s children with CF have the advantages of a higher energy intake than previous generations, a normal to high fat diet and effective pancreatic enzyme supplements. Baseline respiratory function is better maintained and the annual fall in FEV1 values is minimal. Almost certainly they will be at less risk of low BMD than patients who are already adults. The bone coupling process in children with CF has been shown to be similar to that of a control group (Greer et al, 2002). Well children have normal BMD status (Sood et al, 2001; Hardin et al, 2001). We have shown only a low prevalence of reduced BMD in a large unselected population of children with CF (Conway et al, 2008).
Key points
• People with CF have multiple risk factors for low BMD
• The relationship between low BMD and increased fracture risk has not been validated in CF but an association between reduced BMD and fracture is recognised
• It is important to take steps to prevent low BMD
• Bisphosphonate treatment is effective
• BMD should be measured by DXA scan at about 10 years of age and subsequently at three yearly intervals or as determined by clinical need
• Specialist dietitians should review the adequacy of each patient’s nutritional intake at least annually
• Respiratory health should be optimised
• Bisphosphonate treatment should be considered when all other potentially reversible factors for low BMD have been looked for and treated
• Bisphosphonates should only be prescribed in specialist centres
References
Aris RM, Renner JB, Winders AD, et al. Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med 1998; 128: 186-193. [PubMed]
Aris RM, Lester GE, Renner JB, et al. Efficacy of pamidronate for osteoporosis in patients with cystic fibrosis following lung transplantation. Am J Respir Crit Care Med 2000; 162: 941-946. [PubMed]
Aris RM, Ontjes DA, Buell HE, et al. Abnormal bone turnover in cystic fibrosis adults. Osteoporosis Int 2002; 13: 151-157. [PubMed]
Aris RM, Lester GE, Camaniti M, et al. Efficacy of alendronate in adults with cystic fibrosis with low bone density. Am J Respir Crit Care Med 2004; 169: 77-82. [PubMed]
Aris RM, Merkel PA, Bachrach LK, et al. Consensus statement: Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab 2005; 90: 1888-1896. [PubMed]
Bhudhikanok GS, Lim J, Marcus R, et al. Correlates of osteopenia in patients with cystic fibrosis. Pediatrics 1996; 97: 103-111. [PubMed]
Bhudhikanok GS, Wang MC, Marcus R, et al. Bone acquisition and loss in children and adults with cystic fibrosis: a longitudinal study. J Pediatr 1998; 133: 18-27. [PubMed]
Bianchi ML, Romano G, Saraifoger S, et al. BMD and body composition in children and young patients affected by cystic fibrosis. J Bone Miner Res 2006; 21: 388-396. [PubMed]
Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006; 84: 18-28. [PubMed]
Buntain HM, Greer RM, Schluter PJ, et al. Bone mineral density in Australian children, adolescents and adults with cystic fibrosis: a controlled, cross-sectional study. Thorax 2004; 59: 149-155. [PubMed]
Buntain HM, Schluter PJ, Bell SC, et al. Controlled longitudinal study of bone mass accrual in children and adolescents with cystic fibrosis. Thorax 2006; 61: 146-154. [PubMed]
Cadogan J, Eastell R, Jones N, et al. Milk intake and bone mineral acquisition in adolescent girls: Randomised, controlled intervention trial. BMJ 1997; 315: 1255-1260. [PubMed]
Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics 2006; 117: 291-297. [PubMed]
Conway SP, Morton AM, Oldroyd B, et al. Osteoporosis and osteopenia in adults and adolescents with cystic fibrosis: prevalance and associated factors. Thorax 2000; 55: 798-804. [PubMed]
Conway SP, Oldroyd B, Morton A, et al. Effect of oral bisphosphonates on bone mineral density and body composition in adult patients with cystic fibrosis: a pilot study. Thorax 2004; 59: 699-703. [PubMed]
Conway SP, Wolfe SP, Brownlee KG, et al. Vitamin K status among children with cystic fibrosis and its relationship to bone mineral density and bone turnover. Pediatrics 2005; 115: 1325-1331. [PubMed]
Conway SP, Brownlee KG, Wolfe SP, et al. Bone mineral density in an unselected cross section of children with cystic fibrosis. J Cyst Fibros. 2008;7: 469-476. [PubMed]
Cystic Fibrosis Trust Bone Mineralisation Working Group. Bone mineralisation in Cystic Fibrosis. 1st Edition. London. Cystic Fibrosis Trust, February 2007.[Link]
Donovan DS, Papadopoulos A, Staron R, et al. Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med 1998; 158: 1892-1899. [PubMed]
Elkin SL, Burgess J, Hodson ME. Hypovitaminosis D is common in adult patients with cystic fibrosis. Pediatr Pulmonol 1999; Suppl 19: 469.
Elkin SL, Fairney A, Burnett S, et al. Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross sectional study. Osteoporos Int 2001; 12: 366-372. [PubMed]
Elkin SL, Vedi S, Bord S, et al. Histomorphometric analysis of bone biopsies from the iliac crest of adults with cystic fibrosis. Am J Respir Crit Care Med 2002; 166: 1470-1474. [PubMed]
Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr 2004; 144: 253-257. [PubMed]
Gluck O, Colice G. Recognising and treating glucocorticoid-induced osteoporosis in patients with pulmonary diseases. Chest 2004; 125: 1859-1876. [PubMed]
Greer RM, Buntain HM, Wainright C. Uncoupled bone turnover in cystic fibrosis, bone markers and the PTH-vitamin D axis. Pediatr Pulmonol 2002; Suppl 24: 340.
Greer RM, Buntain HM, Potter JM, et al. Abnormalities of the PTH-vitamin D axis and bone turnover markers in children, adolescents and adults with cystic fibrosis: comparison with healthy controls. Osteoporos Int 2003: 14: 404-411. [PubMed]
Hardin DS, Arumugam R, Seilheimer DK, et al. Normal bone mineral density in cystic fibrosis. Arch Dis Child 2001; 84: 363-368. [PubMed]
Haworth CS, Selby PL, Webb AK, et al. Low bone mineral density in adults with cystic fibrosis. Thorax 1999; 54: 961-967. [PubMed]
Haworth CS, Webb AK, Egan JJ, et al. Bone histomorphometry in adult patients with cystic fibrosis. Chest 2000; 118: 434-439. [PubMed]
Haworth CS, Selby PL, Adams JE, et al. Effect of intravenous pamidronate on bone mineral density in adults with cystic fibrosis. Thorax 2001; 56: 314-316. [PubMed]
Janz KF, Gilmour JM, Burns TL, et al. Physical activity augments bone mineral accrual in young children: the Iowa bone development study. J Pediatr 2006; 148: 793-799. [PubMed]
Jones AM, Dodd ME, Webb AK. Acute rib fracture pain in CF Thorax 2001;56:819. [PubMed]
Kalkwarf HJ, Khaury JC, Bean J, et al. Vitamin K, bone turnover and bone mass in girls. Am J Clin Nutr 2004; 80: 1075-1080. [PubMed]
Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention and treatment. J Oral Maxillofac Surg 2005; 63: 1567-1575. [PubMed]
Mischler EH, Chesney PJ, Chesney RW, et al. Demineralisation in cystic fibrosis detected by direct photon absorptiometry. Am J Dis Child 1979; 133: 632-635. [PubMed]
Pike J, Gaskin L, Stewart C. Prevalence of low serum 25-OH vitamin D in adults with cystic fibrosis. Pediatr Pulmonol 2000; Suppl 20: 322.
Rashid M, Durie P, Andrew M, et al. Prevalence of vitamin K deficiency in cystic fibrosis. Am J Clin Nutr 1999; 70: 378-382. [PubMed]
Robinson RF, Nahata MC, Hayes JR, et al. Effectiveness of pre-treatment in decreasing adverse event associated with pamidronate in children and adolescents. Pharmacotherapy 2004; 24: 195-197.
Rovner AJ, Zemel BS, Leonard MB, et al. Mild to moderate cystic fibrosis is not associated with increased fracture risk in children and adolescents. J Pediatr 2005; 147: 327-331. [PubMed]
Schulze KJ, O’Brien KO, Germain-Lee EL, et al. Efficiency of calcium absorption is not compromised in clinically stable pre-pubertal and pubertal girls with cystic fibrosis. Am J Clin Nutr 2003; 78: 110-116. [PubMed]
Schulze KJ, O’Brien KO, Germain-Lee EL, et al. Endogenous fecal losses of calcium compromise calcium balance in pancreatic-insufficient girls with cystic fibrosis. J Pediatr 2003; 143: 765-771. [PubMed]
Schulze KJ, Cutchins C, Rosenstein BJ, et al. Calcium acquisition rates do not support age-appropriate gains in total body bone mineral content in prepuberty and late puberty in girls with cystic fibrosis. Osteoporos Int 2006; 17: 731-740. [PubMed]
Sood M, Hambleton G, Super M, et al. Bone status in cystic fibrosis. Arch Dis Child 2001; 84: 516-520. [PubMed]
Specker BL, Schoenau E. Quantitative bone analysis in children: current methods and recommendations. J Pediatr 2005; 146: 726-731. [PubMed]
Vermeer C. Increased vitamin K intake may decrease bone loss. Osteoporosis Review 2004; 12: 11-14.
Weber P.Vitamin K and bone health. Nutrition. 2001; 17: 880-887. [PubMed]
Wolfe SP, Conway SP, Brownlee KG. Seasonal variation in vitamin D levels in children with cystic fibrosis in the United Kingdom. J Cyst Fibros 2001; p 115.
Wren TA, Liu X, Pitukcheewanout P, et al. Bone densitometry in pediatric populations: discrepancies in the diagnosis of osteoporosis by DXA and CT. J Pediatr 2005; 146: 776-779. [PubMed]
