Cystic Fibrosis Related Diabetes
Introduction
Diabetes mellitus and glucose intolerance are common in adolescent and adult patients with CF (Lanng et al, 1995; Moran et al, 1998). The prevalence of CF related diabetes (CFRD) increases with age (Moran et al, 1998) and in patients over 20 years of age may be as high as 53% (Lanng et al, 1995). CFRD occurs in up to 30% of individuals by the age of 25 and is invariably associated with pancreatic exocrine dysfunction (malabsorption). The average age of onset of CFRD is 18-21 years and it is slightly more common in females (Rosenecker et al, 1995; Yung & Hodson, 1999). Gestational diabetes occurs in between 10 and 30% of pregnancies in women with CF who are not diabetic beforehand (Gilljam et al, 2000; Odegaard et al, 2002; Barak et al, 2005; McMullen et al, 2006). The use of oral corticosteroids will increase the tendency to develop diabetes. CFRD is common among patients referred for lung transplantation and the prevalence of diabetes increases in the years after transplantation related in part to selection of immunosuppressive therapy (Hadjiliadis et al, 2005).
Pathophysiology
CFRD is distinct from type 1 or type 2 diabetes but has features of each of these. It is associated with insulinopenia (low blood insulin levels) and insulin resistance (Hardin et al, 1998; Hardin et al, 1999; Hardin et al, 2001). Ketoacidosis is unusual but can occur, especially if there has been a long period of symptomatic hyperglycaemia before diagnosis (Moran et al, 1998). There is usually enough insulin production to suppress ketogenesis as in type 2 diabetes and glucagon deficiency may also protect from ketone formation.
Histology (microscopic examination) of pancreatic tissue from patients with CF shows destruction of the pancreas by a process of fatty replacement and fibrosis (scarring). This leads to a progressive loss in the number and function of secretory cells. There may be a 50% reduction in ß-cells within the islets of Langerhans which produce insulin. These changes are likely to result from obstruction of pancreatic ducts by abnormal, thick secretions. Autopsy studies, however, have not shown that fibrotic islet changes are significantly greater in patients with CFRD than in patients with CF without diabetes. Such studies suggest that CFRD is more than merely severe fibrosis induced islet damage.
Islet amyloid polypeptide is co-secreted with insulin in normal health but does not accumulate within the islet. Amyloid deposition is not seen in type 1 diabetes or in chronic pancreatitis but is seen in type 2 diabetes. Autopsy studies did not find islet amyloid in any patients with CF without CFRD but this deposition was found in 17% of the borderline diabetic cases and in 69% of the cases with full CFRD. There is thus a suggested similarity between CFRD and type 2 diabetes. This has led to speculation that impaired glucose tolerance is very common in adults with CF because of fibrotic damage to the islet, but may only progress to full CFRD in patients with a genetic defect in insulin secretion similar to that found in type 2 diabetes (Moran et al, 1998).
Diabetes mellitus and impaired glucose tolerance in CF is associated with a delay and reduction in peak insulin production following a carbohydrate load. In CF there also appears to be an element of insulin resistance and the normal anabolic effect of insulin is reduced (Holl et al, 1997; Hardin et al, 1998).
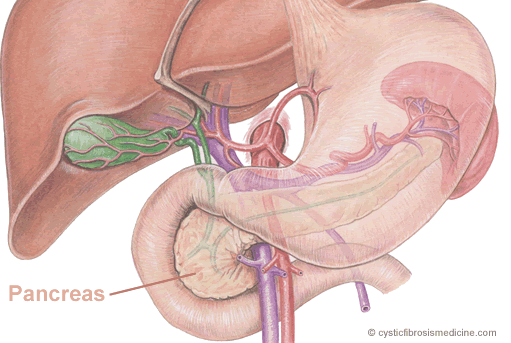
Figure 1: Diagram showing the anatomy of the pancreas which can be seen behind the stomach. The pancreas has two very important functions. Firstly it produces pancreatic digestive juices (the exocrine pancreas) and secondly it produces insulin and other hormones (endocrine pancreas).
Impact of diabetes on pulmonary function and weight
The insulin deficient state leads to worse pulmonary outcome (Milla et al, 1999; Milla et al, 2000; Koch et al, 2001). The early diagnosis of, and intervention in diabetes mellitus can have a profound impact on patient well being, protecting against weight loss and deterioration in lung function (Lanng et al, 1992). The negative impact of diabetes on pulmonary status in CF appears greater in female patients than male patients (Sims et al, 2005; Milla et al, 2005).
Screening tests for diabetes
Fasting glucose levels do not reliably identify CFRD (Lanng, 1997; Baker & DuBois, 1999) even if impaired fasting glucose is used as an indication for OGTT (Mueller-Brandes et al, 2005). HbA1C has been used as a screening test but is severely limited as levels are often normal at the time of diagnosis of CFRD (Etherington et al, 2000; Verma et al, 2002). We believe that patients over 10 years of age should have an annual oral glucose tolerance test (OGTT) as it is the only sure way of detecting diabetes mellitus (Etherington et al, 2000; Verma et al,2002). An OGTT should also be part of an investigative screen for unexplained weight loss or deterioration in lung function.
At the adult unit we have carried out more than 1,200 OGTTs since 1995. Impaired glucose tolerance is common (approximately 23% of all the OGTTs we have performed) and may progress to full diabetes or revert to normal. We recommend a repeat OGTT in such patients after a six month interval. A diabetic glucose tolerance test (approximately 12% of all the OGTTs we have performed) does not always mean that the person has clinical diabetes. In some patients a diabetic glucose tolerance test will revert to normal or become impaired (Lanng et al,1995). Interestingly, approximately 5% of all the OGTTs we have performed show a two hour blood glucose level below the normal limit. The significance of this is unknown although some patients do report symptomatic hypoglycaemia (low blood sugars) after meals. As there is an increased incidence of CFRD in adults it is essential that the OGTT is carried out accurately and is as reproducible as possible hence there are minor differences between the way the OGTT is carried out between the paediatric and adult units.
We recommend pre and post prandial blood glucose monitoring in patients with a diabetic OGTT result, with post prandial blood glucose possibly being of most relevance. If blood glucose levels are normal we recommend a repeat OGTT after six months. If blood glucose levels are high we start insulin therapy. At large adult clinics there are obvious practical problems in performing an annual OGTT on all non diabetic patients. The Brompton group suggest selective OGTT screening in patients with abnormal HbA1C or random blood glucose results, symptoms of hyperglycaemia or weight loss (Yung et al, 1997). We continue to rely on the OGTT but acknowledge that not all patients actually undergo an OGTT every year. In the period 1995-1999 on average 48% were missed each year (Etherington et al, 2000). Most patients were only screened every two years. As a consequence of this study, improved communication and documentation of OGTTs was implemented and our latest data show that between 2004 and 2006 despite increasing patient numbers on average 32% (27-35%) of non-diabetic patients did not have an annual OGTT.
Continuous subcutaneous glucose monitoring seems to offer no advantage over technically successful OGTT in the detection of CFRD (Jefferies et al, 2005).
Treatment of diabetes
Following diagnosis some patients can be controlled for some time with oral treatment with insulin secretagogue agents which stimulate insulin secretion from the patient’s own pancreas (Rosenecker et al, 2001). This treatment is not as effective as insulin therapy (Moran et al, 2001). A Cochrane review of insulin versus oral glucose-lowering therapy for CFRD found no studies of sufficient quality to draw helpful conclusions (Onady & Stolfi, 2005). Given that the predominant underlying cause of hyperglycaemia is insulin insufficiency there has been little enthusiasm and no available data on the use of the insulin sensitising thiazolidinedione (glitazone) agents in CFRD. Metformin is not used for similar reasons and due to the high rates of bowel side-effects.
Insulin therapy is our treatment of choice. The introduction of insulin therapy in CFRD improves respiratory function and nutritional status (Nousia Arvanitakis et al, 2001; Rolon et al, 2001). Many patients may only show significant hyperglycaemia after meals. Thrice daily soluble insulin is useful in such cases. The new rapid acting insulin analogues can be given with or after a meal and because of their short duration of action they are associated with a lower risk of hypoglycaemia. Treatment with insulin to cover meals may also protect against endogenous protein breakdown and favour protein synthesis in the fed state (Moran et al, 2001). New long-acting insulin analogues have been useful in our practice for patients with fasting hyperglycaemia. Continuous subcutaneous insulin infusion therapy has been used successfully in a few patients with CFRD (Sulli & Shashaj, 2003; Reali et al, 2006).
All patients should receive individualised dietary review and advice at the time of the diagnosis of CFRD. They should usually maintain a high energy diet and the insulin dose should be tailored to their individual requirements. They should not decrease their carbohydrate intake but should be encouraged to eat regular meals with similar carbohydrate content each day.
Nasogastric or gastrostomy feeding may be necessary in patients with CF and CFRD. Monitoring of blood sugars pre and post feed should be routine practice to assess any effect of enteral tube feeding on nocturnal blood sugar levels. Some patients do develop high sugar levels overnight (nocturnal hyperglycaemia), (Smith et al, 1994; Etherington et al, 2000) and may require insulin just to cover their overnight feed. In patients known to have CFRD the introduction of enteral tube feeding should be closely monitored and insulin therapy adjusted accordingly. Education about practical problems such as pump failure following the injection of insulin, vomiting etc, is provided (Cystic Fibrosis Trust, 2004).
Collateral effects of diabetes
Work in the Leeds and Manchester Adult CF Units comparing eating behaviour and attitudes, body satisfaction and self esteem in patients with CFRD and non-diabetic adults with CF found no difference between the groups relating to actual, perceived or desired body mass index. However, those with CFRD reported a greater number of problems concerning food/eating behaviours with female diabetics reporting significantly more problems than males. Both male and females with CFRD were less satisfied with their body appearance than controls. The method of treatment was also important. Patients treated with insulin reported greater problems with food/eating behaviours than those taking oral medication (Abbott et al, 1998).
The additional burden of CFRD needs to be acknowledged in patient management and care.
Complications of diabetes
Late complications of diabetes – diabetic retinopathy, microalbuminuria, diabetic nephropathy and neuropathy (damage to the eyes, kidneys and nerves) have been reported in patients with CF (Lanng et al, 1994; Yung et al, 1998; Scott et al, 2000; Andersen et al, 2006). Blood glucose control as measured by HbA1C is related to the risk of long- term complications in type 1 diabetes (DCCT Trial, 1993). There is no reason to think that the risk in CFRD is different (Wilson et al, 2000). We aim for tight blood sugar control to reduce the risk of long term diabetic complications. We offer annual screening for diabetic complications and a full diabetic review by our Consultant Diabetologist to all our patients with CFRD. Screening for retinopathy should comply with the national standards for retinopathy screening in all people with diabetes. Screening for nephropathy is more complex as the significance of microalbuminuria (small amounts of protein in the urine) in CFRD is less clear. Co-existant infection can increase urinary albumin excretion into the pathological microalbuminuria range. The reduced muscle mass common in patients with CF results in reduced urinary creatinine which tends to elevate the urinary albumin:creatinine ratio, giving false positive results (Dobson et al, 2005).
Key points
•The prevalence of CFRD increases with age and occurs in up to 30% of people with CF by the age 25 years of age
• An oral glucose tolerance test is the only reliable screening test for CFRD
•A diabetic oral glucose tolerance test result on its own does not necessarily mean that some one has developed CFRD
• Insulin therapy is the treatment of choice
• Patients are at risk of developing diabetic complications and good diabetic control is imperative
• Patients with CFRD should maintain a high energy diet recommended for CF and any dietary modification should only be undertaken when supervised by a CF specialist dietitian
References
Abbott J, Conway SP, Etherington C, et al. Cystic fibrosis related diabetes, eating behaviors and body satisfaction. Pediatr Pulmonol 1998; Suppl 17: 395.
Andersen HU, Lanng S, Pressler T, et al. Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006; 29: 2660-2663. [PubMed]
Baker RD, DuBois KD. Screening patients with CF for abnormalities in glucose regulation. Pediatr Pulmonol 1999; Suppl 19: 304
Barak A, Dulitzki M, Efrati O, et al. Pregnancies and outcome in women with cystic fibrosis. Isr Med Assoc J 2005; 7: 95-98. [PubMed]
Cystic Fibrosis Trust Diabetes Working Group. Management of Cystic Fibrosis Related Diabetes Mellitus. London. Cystic Fibrosis Trust, June 2004. [link]
Culler FL, McKean LP, Buchanan C et al. Glipizide treatment of patients with cystic fibrosis and impaired glucose tolerance. J Pediatr Gastroenterol Nutr 1994; 18: 375-378. [PubMed]
DCCT. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependant diabetes mellitus. N Engl J Med 1993; 329: 977-986. [PubMed]
Dobson L, Stride A, Bingham C, et al. Microalbuminuria as a screening tool in cystic fibrosis-related diabetes. Pediatr Pulmonol 2005; 39: 103-107. [PubMed]
Etherington C, Morton A, White H, et al. Screening for CFRD – 5 years experience using the OGTT in a Regional Adult Unit. Abstract Book, XIIIth International Cystic Fibrosis Congress Conference Proceedings, 2000: 134.
Gilljam M, Antoniou M, Shin J, et al. Pregnancy in cystic fibrosis. Fetal and maternal outcome. Chest 2000; 118: 85-91. [PubMed]
Hadjiliadis D, Madill J, Chaparro C, et al. Incidence and prevalence of diabetes mellitus in patients with cystic fibrosis undergoing lung transplantation before and after lung transplantation. Clin Transplant 2005; 19: 773-778. [PubMed]
Hardin DS, LeBlanc A, Lukenbaugh, S et al. Proteolysis associated with insulin resistance in cystic fibrosis. Pediatr 1998; 101: 433-437. [PubMed]
Hardin DS, LeBlanc A, Para L, et al. Hepatic insulin resistance and defects in substrate utilisation in cystic fibrosis. Diabetes 1999; 48: 1082-1087. [PubMed]
Hardin DS, LeBlanc A, Marshall G, et al. Mechanisms of insulin resistance in cystic fibrosis. Am J Physiol Endocrinol Metab 2001; 281: 1022-1028. [PubMed]
Holl RW, Heinze E, Wolf A, et al. Reduced pancreatic insulin release and reduced peripheral insulin sensitivity contribute to hyperglycaemia in cystic fibrosis. Eur J Pediatr 1995; 154: 356-361. [PubMed]
Holl RW, Wolf A, Thon A, et al. Insulin resistance with altered secretory kinetics and reduced proinsulin in cystic fibrosis patients. J Pediatr Gastroenterol Nutr 1997; 25: 188-193. [PubMed]
Jefferies C, Solomon M, Perlman K, et al. Continuous glucose monitoring in adolescents with cystic fibrosis. J Pediatr 2005; 147: 396-398. [PubMed]
Koch C, Rainisio M, Madessani U, et al. Presence of cystic fibrosis related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001; 32: 343-350. [PubMed]
Lanng S, Thorsteisson B, Nerup J, et al. Influence of the development of diabetes mellitus on the clinical status in patients with cystic fibrosis. Eur J Paediatr 1992; 151: 684-687. [PubMed]
Lanng S, Thorsteinsson B, Lund-Andersen C, et al. Diabetes mellitus in Danish cystic fibrosis patients: prevalence and late complications. Acta Paediatr 1994; 83: 72-77. [PubMed]
Lanng S, Hansen A, Thorsteinsson B, et al. Glucose tolerance in patients with cystic fibrosis: five year prospective study. BMJ 1995; 311: 655-659. [PubMed]
Lanng S. Glucose intolerance in cystic fibrosis. Dan Med Bull 1997; 44: 23-39. [PubMed]
McMullen AH, Pasta DJ, Frederick PD, et al. Impact of pregnancy on women with cystic fibrosis. Chest 2006; 129: 706-711. [PubMed]
Milla CE, Moran AM. Insulin production at baseline influences, the rate of decline in FEV1 in CF patients without fasing hyperglycaemia. Pediatr Pulmonol 1999; Suppl 19: 489.
Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 2000; 162: 891-895. [PubMed]
Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005; 28: 2141-2144. [PubMed]
Moran A, Doherty L, Wang X, et al. Abnormal glucose metabolism in cystic fibrosis. J Pediatr 1998; 133: 10-17. [PubMed]
Moran A, Hardin D, Rodman D, et al. Diabetes mellitus in patients with cystic fibrosis: the impact of diabetes mellitus on pulmonary function and clinical outcome. Eur J Med Res 2001; 6: 345-350.
Moran A, Milla C, Ducret R, et al. Protein metabolism in clinically stable adult cystic fibrosis patients with abnormal glucose tolerance. Diabetes 2001; 50: 1336-1343. [PubMed]
Moran A, Phillips J, Milla C. Insulin and glucose excursion following premeal Lispro or Repaglinide in cystic fibrosis related diabetes. Diabetes Care 2001; 24: 1706-1710. [PubMed]
Mueller-Brandes C, Holl RW, Nastoll M, et al. New criteria for impaired fasting glucose and screening for diabetes in cystic fibrosis. Eur Respir J 2005; 25: 715-717. [PubMed]
Nousia-Arvanitakis S, Galli-Tsinopolou A, Karamouzis M. Insulin improves clinical status of patients with cystic fibrosis related diabetes. Acta Pediatr 2001; 90: 515-519. [PubMed]
Odegaard I, Stray-Pedersen B, Hallberg K, et al. Maternal and fetal morbidity in pregnancies of Norwegian and Swedish women with cystic fibrosis. Acta Obstet Gynecol Scand 2002; 81: 698-705. [PubMed]
Onady GM, Stolfi A. Insulin and oral agents for managing cystic fibrosis-related diabetes. Cochrane Database Syst Rev 2005; 3: CD004730. [PubMed]
Reali MF, Festini F, Neri AS, et al. Use of continuous subcutaneous insulin infusion in cystic fibrosis patients with cystic fibrosis-related diabetes awaiting transplantation. J Cyst Fibros 2006; 5: 67-68. [PubMed]
Rolon MA, Benali K, Munck A, et al. Cystic fibrosis related diabetes: clinical impact of prediabetes and effects of insulin therapy. Acta Pediatr 2001; 90: 860-867. [PubMed]
Rosenecker J, Eichler I, Kuhn L, et al. Genetic determinants of diabetes mellitus in patients with cystic fibrosis. J Pediatr 1995; 127: 441-443. [PubMed]
Rosenecker J, Eichler I, Barmeier H, et al. Diabetes mellitus and cystic fibrosis: comparison of clinical parameters in patients treated with insulin versus oral glucose lowering agents. Pediatr Pulmonol 2001; 332: 351-355. [PubMed]
Scott AI, Clarke BE, Healy H, et al. Microvascular complications in cystic fibrosis related diabetes mellitus: a case report. JOP 2000; 1: 208-210. [PubMed]
Sims EJ, Green MW, Mehta A. Decreased lung function in female but not male subjects with established cystic fibrosis-related diabetes. Diabetes Care 2005; 28: 1581-1587. [PubMed]
Smith DL, Clarke JM, Stableforth DE. A nocturnal nasogastric feeding programme in cystic fibrosis adults. J Hum Nutr Diet 1994; 7: 257-262.
Sulli N, Shashaj B. Continuous subcutaneous insulin infusion in children and adolescents with diabetes mellitus: decreased HbA1C with low risk of hypoglycemia. J Pediatr Endocrinol Metab 2003; 16: 393-399. [PubMed]
Verma A, Claridge A, Havelock T, et al. Re-audit of the screening protocol for cystic fibrosis related diabetes (CFRD) in an adult centre. Thorax 2002; 57(Suppl 11): 20.
Verma A, Das M, Ahluwalia A, et al. Gestational diabetes in cystic fibrosis. J Cyst Fibros 2002; 1: S155-S156.
Wilson DC, Kalnins D, Stewart C, et al. Challenges in the dietary management of cystic fibrosis related diabetes mellitus. Clin Nutr 2000, 19: 87-93. [PubMed]
Yung B, Kemp M, Hooper J, et al. Diagnosis of cystic fibrosis related diabetes: a selective approach in performing the oral glucose tolerance test based on a combination of clinical and biochemical criteria. Thorax 1997; 54: 40-43. [PubMed]
Yung B, Landers A, Mathalone B et al. Diabetic retinopathy in adult patients with cystic fibrosis related diabetes. Respir Med 1998; 92: 871-872. [PubMed]
Yung B, Hodson ME. Diabetes in cystic fibrosis. J R Soc Med 1999; 92 (Suppl 37): 35-40. [PubMed]
