DNase and cystic fibrosis
Introduction
In the 1950s research established that purulent lung secretion from patients with cystic fibrosis (CF) contained large amounts of deoxyribonucleic acid (DNA) (Chernick & Guilio, 1959; Potter et al, 1960; Shak et al, 1990). These high levels of extra cellular DNA are released from degenerating neutrophils (white cells) and accumulate within the airways in response to either chronic infection or innate inflammation, resulting in the production of sputum with a high viscoelasticity.
Chronic airway inflammation can be demonstrated at an early age, even in the absence of demonstrable infection (Armstrong et al, 1995; Armstrong et al, 1996; Armstrong et al, 1997; Kahn et al, 1995). Studies have also shown increased DNA levels in bronchoalveolar lavage fluid obtained from infants with CF (Kirchner et al, 1996).
The combination of viscous secretion and a heightened inflammatory response within CF lungs leads to airway obstruction, progressive bronchiectasis and eventually, end stage respiratory failure.
The history of DNase
A number of studies in the 1950s demonstrated that sputum viscoelasticity in patients with suppurative lung disease could be reduced with bovine pancreatic Dornase which enzymatically cleaves extra cellular DNA into shorter molecules (Armstrong et al, 1950; Elmes et al, 1953; Salomon et al, 1954; Spier et al, 1961). This has the effect of converting thick gel like sputum into liquid (Shak et al, 1990). Clinical studies were carried out in patients with a range of respiratory conditions including asthma, chronic bronchitis, pneumonia, atelectasis and bronchiectasis (Armstrong et al, 1950; Elmes et al, 1953; Salomon et al, 1954; Spier et al, 1961). Treatment appeared to be more effective in patients with large amounts of tenacious mucopurulent secretions. More importantly, Chernick et al demonstrated in 1959 that infected tracheobronchial secretions collected from patients with CF had a higher DNA concentration when compared to patients with non-CF bronchiectasis (Chernick et al, 1959). This had significant implications as it suggested that DNase therapy could potentially be more effective in CF patients. Unfortunately, however, the use of bovine DNase had to be abandoned due to the occurrence of occasional severe adverse reactions such as bronchospasm and acute respiratory distress (Raskin, 1968).
In the 1990s recombinant human DNase (rhDNase) was successfully produced, following the cloning and sequencing of the human enzyme DNase. It was shown to improve in-vitro the rheological properties of purulent sputum from CF by cleaving DNA derived from human neutrophils (Shak et al, 1990). It was as effective as bovine DNase but without the serious side effects. A large number of clinical studies assessing the safety and effectiveness of rhDNase in patients with CF have since been carried out and confirm the initial findings.
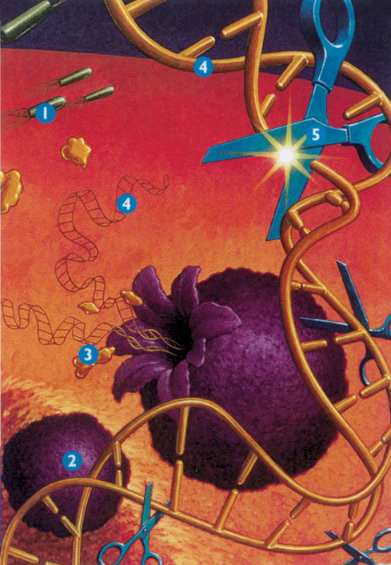
Figure 1: DNase which enzymatically cleaves extra cellular DNA into shorter molecules 1. Bacteria grow in the mucous, causing inflammation, 2. Neutrophils are sent in response to inflammation, 3. As neutrophils die, elastase is released which damages lung cells, 4. DNA is also released, which thickens mucous, 5. Pulmozyme reduces the thickness of the mucous by breaking down DNA.
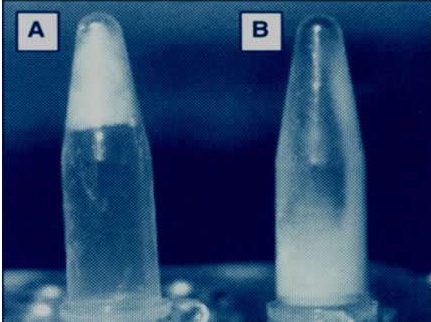
Figure 2: The addition of DNase to sputum has the effect of converting thick gel like sputum (A) into liquid (B)
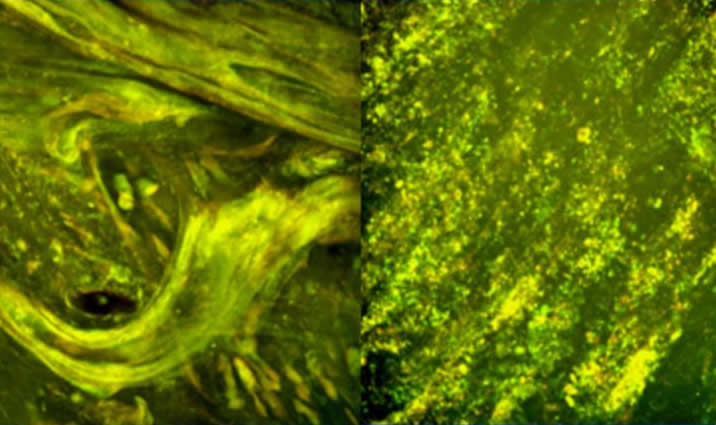
Figure 3: Image showing DNase depolymerizing sputum DNA filaments
Evidence for use of rhDNase
rhDNase is a safe and effective treatment in patients with mild, moderate or severe respiratory disease (Fuchs et al, 1994; Shah et al, 1995; Harms et al, 1998; Henry et al, 1998). In the more severely ill patients longer term therapy may be needed before a beneficial effect is seen (Hodson, 1995). A maximal improvement in FEV1 of about 5-13% is seen. This occurs within the first few months of treatment. After this initial peak response, lung function will plateau at a lower level of improvement (Fuchs et al, 1994; Shah et al, 1995; Harms et al, 1998; Quan et al, 2001). rhDNase therapy has little effect on overall exercise capacity but can result in significant improvements in a subset of patients different to those who show the best outcome in resting pulmonary function (Barker et al, 2004). A minority of patients complain of a hoarse voice which usually settles with continued treatment. The drug is well tolerated by most patients independent of the severity of lung disease (Fuchs et al, 1994; Hodson, 1995).
In the early trials although there was a significant increase in mean FEV1 values individual patient responses varied a lot. About 6% of patients had a fall in respiratory function with treatment. In the paediatric unit at the Brompton after treatment for one year a third of children showed a greater than 20% increase in FEV1 and a third showed a fall in respiratory function. Other units, including our own, have had similar experience. Importantly, some of the children with worse respiratory function on treatment said that they felt better (Davies et al, 1997). There is unfortunately no way of pre-determining responders. Patients receiving rhDNase must be properly monitored, preferably by CF centre teams. As highlighted in the Brompton study, merely feeling better is not enough (Bush, 1998).
Longer term treatment
Shah et al compared a cohort of patients with CF who had been treated with rhDNase with matched controls (Shah et al, 2001). The study demonstrated that treatment resulted in a relative reduction in the number of infective exacerbations per patient per year over four years. Requirements for antibiotic therapy were also significantly higher in the control group, suggesting that rhDNase may have a long term influence on disease progression. Although the results should be interpreted with caution as the number of patients was small, similar results were found when analyzing a large cohort of patients (n = 2,023) from the Epidemiological Registry of CF (Hodson et al, 2003). Patients receiving rhDNase had 25 fewer exacerbations per 100 treated patients per year when compared to those not receiving treatment. Untreated patients also showed a decline in FEV1 (-2.3%) over a two year period while treated patients tended to remain stable (+0.3%).
Shah’s case control study failed to show a statistical difference in the rate of decline in FEV1 with treatment (Shah et al, 2001). Konstan et al using data from the Epidemiologic Study of CF, showed a reduction in the mean rate of decline in lung function of 46% over a two year period in rhDNase treated patients, with no change in the control group (Konstan et al, 2006).
rhDNase therapy over a 12 month period is associated with a significantly lower yield of potential lower respiratory tract pathogens (Frederiksen et al, 2006). The most striking difference was seen for S. aureus with a 12 month prevalence of 30% in the untreated group compared to 16% in the treated group. There was no significant change in P. aeruginosa prevalence. The lower infection rate may be associated with less respiratory exacerbations and inflammatory activity with better preservation of lung function.
Young patients and patients with mild disease
We believe in the concept that early treatment before chronic inflammation has led to irreversible lung damage is effective treatment. Wagener et al showed that rhDNase can be delivered to the airways of young children in amounts comparable to those achieved with older children and with similar efficacy (Wagener et al, 1998). Nasr et al showed significant improvements in chest high resolution CT scores in a randomised, double-blind, placebo-controlled trial in 12 children less than five years old over 100 days of treatment (Nasr et al, 2001). In a multicentre, randomised, placebo-controlled, two year study investigating rhDNase therapy in young patients with CF (mean age 8.4 years) and good lung function (mean FEV1, FVC and FEF25-75 were 95%, 102% and 85% predicted respectively), patients randomised to active therapy had an initial increase in FEV1 that was sustained above that of the placebo group for the duration of the study (Quan et al, 2001). At 96 weeks patients on the treatment limb had maintained FEV1 at their baseline values, whereas patients in the placebo group had a mean decrease of 3.2% in FEV1 from baseline. A similar but more sustained pattern was seen with FEF25-75, suggesting that rhDNase preserves small airway function. Treatment resulted in a 34% reduction in the risk of respiratory tract exacerbations. The recent analysis of a large cohort of patients from the Epidemiological Registry of CF also supports the use of rhDNase in young patients and those with mild disease (Hodson et al, 2003). rhDNase inhalation before airway clearance therapy improves airway patency in children with CF (van der Giessen et al, 2007). Based on these results we offer rhDNase to all our patients, including symptom free children without P. aeruginosa infection. As long as treatment is tolerated it is continued. Newly diagnosed infants are treated as soon as nebulised therapy is tolerated.
Airway inflammation
Some studies have suggested that rhDNase may increase airway inflammation by releasing pro-inflammatory cytokines that are bound to DNA in airway secretions. Other studies do not confirm this or, more importantly, suggest that rhDNase may even downgrade airway inflammation. Suri et al did not show any significant difference in the changes in interleukin 8 (IL-8), eosinophilic protein, myeloperoxidase or neutrophil elastase levels with either hypertonic saline or rhDNase therapy (Suri et al, 2002).
Paul et al used bronchoalveolar lavage to more accurately determine the degree of airway inflammation. In a three year study, 105 patients less than five years of age and with mild CF were randomised to receive either rhDNase or no rhDNase (Paul et al, 2004). Patients with a normal percentage neutrophil count in the baseline bronchoscopy lavage sample served as controls. Repeat bronchoscopy was performed at 18 and 36 months. rhDNase treatment stabilized neutrophilic airway inflammation in the treatment group whereas the percentage neutrophils in the untreated and control groups increased. Total interleukin-8 (IL-8) levels increased in the untreated group but remained stable in the other two groups. This study shows that neutrophil airway inflammation increases over time, even in children with normal lung function. rhDNase treatment protects against this increase and may therefore protect against inflammatory damage in the airways. Ratjen and colleagues showed that 18 months rhDNase treatment in children and young adults with normal lung function significantly reduced bronchoscopy lavage DNA concentrations. The resulting increased mucociliary clearance and removal of retained secretions and neutrophil degredation products from the lower airways may indirectly reduce airway inflammation (Ratjen et al, 2005).
rhDNase dosage, nebulisers and compressors; general precautions
There is no evidence that increasing the dose above 2.5 mg daily achieves any greater therapeutic effect, although twice daily dosing is sometimes tried as a short term measure with intense physiotherapy for patients with tenacious secretions associated with an acute respiratory exacerbation. Alternate day dosing has been suggested (Suri et al, 2002). Short term bronchoscopic administration to try and relieve lobar or segmental collapse due to retained secretions may be indicated (Touleimat et al, 1995). We have had only variable success with this method.
It is essential that rhDNase is not mixed with any other drug in the nebuliser chamber. It should not be taken within an hour of an inhaled antibiotic as the latter may denature the protein structure of rhDNase, and not less than an hour before chest physiotherapy.
The sequence of treatments is tailored to the patient. The following is usual but may be adapted according to lifestyle and patient response to rhDNase: (a) bronchodilators, (b) physiotherapy, (c) nebulised antibiotics and (d) rhDNase (at least 60 minutes after inhaled antibiotics). A number of delivery devices are available for use with rhDNase. With conventional compressor systems the compressor flow should not be too high. Therefore caution must be exercised when prescribing a compressor for use with both nebulised antibiotics and rhDNase. Roche do not have evidence to suggest a maximal air flow rate for use with rhDNase. They recommend a flow rate of 6.5 L/min when pressurized oxygen is used. However, we do not recommend oxygen as a driving gas for nebulisation. rhDNase can also be delivered with the new membrane technology nebulisers e.g. the eFlow® (PARI) or the I-neb® (Respironics). Individual treatment regimens should be established.
rhDNase is heat sensitive and should be kept in a refrigerator but may be taken out of the refrigerator e.g. when travelling, for approximately 10 hours. If the time out of the refrigerator exceeds 10 hours the drug should be discarded as it will no longer be effective. Cool bags/packs may be used for the transportation of rhDNase if required.
Sanders et al suggest that non-response to rhDNase in some patients might be associated with low sputum magnesium levels. This observation requires confirmation in further studies and may have important clinical implications (Sanders et al, 2006).
Controversies in rhDNase use
Some physicians question the long term effect of rhDNase treatment. Results from a large North American CF Centre showed that respiratory function decreased more in the two years after starting rhDNase therapy than in the two years preceding, and there was no change in hospital admission rates (Milla et al, 1998). Treatment may be just cosmetic, masking an on-going destructive process in the lungs. When treatment was stopped patients’ respiratory function fell below their previous baseline (Zach, 1996). Continuing improvements in lung function tests after two years of rhDNase may coexist with deteriorating high resolution CT scan appearances. Improvement in lung function may therefore not prevent on-going lung destruction (Roos-Liegmann et al, 1999).
On the other hand Geller showed that rhDNase improves lung function in patients with mild lung disease and near normal function (Geller, 1997), and Rittie et al showed prevention of pulmonary deterioration in most patients over four years of treatment (Rittie et al, 1999). An audit of our own treated adult patients showed a 5% benefit in percent predicted FEV1 at one year. This differential was maintained through the study period to three years and associated with a reduced need for intravenous antibiotic treatments (Ratnalingam et al, 2001). We believe that rhDNase is effective and offers a small long term advantage (Geddes & Shah, 1999; Shah et al, 2001).
Alternate day and daily rhDNase administration were compared in a prospective, open labelled study in children. Both regimens showed a similar improvement in FEV1. The authors concluded that rhDNase could be given on alternate days as its effect lasts for at least 48 hours (Suri et al, 2002). It is important to recognise that this study was conducted over relatively short consecutive 12 week periods and that secondary outcomes such as the number of respiratory exacerbations, weight gain, quality of life and exercise tolerance may need a longer study to show differences. We do not believe that alternate day therapy can be recommended on the basis of this single, short term study.
Bronchoscopic instillation of rhDNase
Bronchoscopic instillation of human recombinant DNase can be effective in patients with focal atelectasis or lobar collapse where resolution has proved resistant to standard medical therapy. Slattery et al reported the successful bronchoscopic administration of 2.5 mg rhDNase in 10 ml of normal saline in three patients with CF with persistent lobar atelectasis due to mucous plugging (Slattery et al, 2001).
Adverse effects and anti-rhDNase antibody production
Long term studies in patients with CF have demonstrated that nebulised rhDNase has a good safety profile, although adverse effects have been reported (Shah et al, 1995). Eisenberg et al carried out a study specifically designed to determine the effect of repeated intermittent courses of high dose (10 mg) aerosolized rhDNase twice daily for 24 weeks (eight times the standard dose) on the development of adverse reactions (Eisenberg et al, 1997). Anti-rhDNase antibodies developed in 8.7% of patients, but sero positive results were not associated with clinical deterioration. Lower doses of rhDNase are also associated with the production of low levels of IgG antibodies compared to rhDNase in around 2-4% of patients ( Aitken et al, 1992; Fuchs et al, 1994; Wagener et al, 1998).
Adverse effects to rhDNase therapy have been reported. These include increased bronchial reactivity (particularly at very high doses), rash, chest pain, conjunctivitis, rhinitis, pharyngitis, and voice alteration (Fuchs et al, 1994; Eisenberg et al , 1997; Wagener J, 1998; Burrows et al, 2002). In a large randomized, double-blind, placebo-controlled study by Fuchs et al, 2.5 mg of rhDNase administered once or twice daily for 24 weeks resulted in significantly higher levels of voice alteration and laryngitis when compared to placebo, but this was rarely severe and tended to resolve within 21 days of onset (Fuchs et al, 1994). A slightly higher frequency of fever, decrease in FVC (>10% predicted), rhinitis and dyspepsia have also been reported in patients with advanced lung disease receiving DNase as compared to placebo (McCoy et al, 1996).
Haemoptysis
There is no clear evidence that rhDNase directly causes haemoptysis, although treatment should be halted in those patients with moderate to significant bleeding as it may exacerbate the situation by dislodging mucous plugs.
Adherence
There is variation in adherence to rhDNase. Patient self-reporting is likely to over estimate drug usage. Close monitoring of patients and continued assessment of their compliance should be carried out during routine clinic visits and annual assessments.
Key points
• DNase reduces sputum viscosity (stickiness)
• DNase is effective and safe at all stages of lung disease
• DNase is effective with long term use
• Probably protects against the damaging effects of lung inflammation
Recommendation
• DNase should be used as soon as possible after diagnosis and offered to all patients
References
Aitken ML, Burke W, McDonald G, et al. Recombinant human DNase inhalation in normal subjects and patients with cystic fibrosis. JAMA 1992; 267: 1947-1951. [PubMed]
Armstrong JB, White JC. Liquifaction of viscous purulent exudates by deoxyribonuclease. Lancet 1950: 739-742. [PubMed]
Armstrong DS, Grimwood K, Carzino R, et al. Lower respiratory tract infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995; 310: 1571-1572. [PubMed]
Armstrong DS, Grimwood K, Carlin JB, et al. Bronchoalveolar lavage and oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol 1996; 21: 267-275. [PubMed]
Armstrong DS, Grimwood K, Carlin JB, et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Resp Crit Care Med 1997; 156: 1197-204. [PubMed]
Barker M, Franke E, Bohle M, et al. Effect of DNase on exercise capacity in cystic fibrosis. Pediatr Pulmonol 2004; 38: 70-74. [PubMed]
Burrows JA, Bunting JP, Masel PJ, et al. Nebulised dornase alpha: adherence in adults with cystic fibrosis. J Cystic Fibrosis 2002;1:255-259. [PubMed]
Bush A. Early treatment with dornase alpha in cystic fibrosis: what are the issues? Pediatr Pulmonol 1998; 25: 79-82. [PubMed]
Chernick WS, Barbero GJ. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics 1959: 24: 739-45. [PubMed]
Davies J, Trindade MT, Wallis C, et al. Retrospective review of the effects of rhDNase in children with cystic fibrosis. Pediatr Pulmonol 1997; 23: 243-248. [PubMed]
Eisenberg JD, Aitken ML, Dorkin HL, et al. Safety of repeated intermittent courses of aerosolized recombinant human deoxyribonuclease in patients with cystic fibrosis. J Pediatr 1997; 131: 118-124. [PubMed]
Elmes PC, White JC. Deoxyribonuclease in the treatment of purulent bronchitis. Thorax 1953; 8: 295-230. [PubMed]
Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New Engl J Med 1994; 331: 637-642. [PubMed]
Frederiksen B, Pressler T, Hansen A, et al. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr 2006; 95: 1070-1074. [PubMed]
Geddes DM, Shah PL. Where we are now with rhDNase. Lancet. 353: 1727-1727. [PubMed]
Geller DE. Aerosolised dornase alpha in cystic fibrosis: is there a role in the management of patients with early obstructive lung disease? Pediatr Pulmonol 1997; 24: 155-158. [PubMed]
Harms HK, Matouk E, Taurnier G, et al. Multicenter, open-label study of recombinant human DNase in cystic fibrosis patients with moderate lung disease. DNase International Study Group. Pediatr Pulmonol 1998; 26: 155-161. [PubMed]
Henry R L, Gibson P G, Carty K , et al. Airway inflammation after treatment with aerolised deoxyribonuclease in cystic fibrosis. Pediatr Pulmonol 1998; 26: 97-100. [PubMed]
Hodson ME. Clinical studies of rhDNase in moderately and severely affected patients with CF – overview. Respiration 1995; 62 (suppl 1): 29-32. [PubMed]
Hodson ME. Aerosolized dornase alfa (rhDNase) for therapy of cystic fibrosis. Am J Respir Crit Care Med 1995;151(3 Pt 2):S70-4. [PubMed]
Hodson ME, McKenzie S, Harms HK, et al. Dornase alpha in the treatment of cystic fibrosis in Europe: A report from the Epidemiologic Registry of cystic fibrosis. Pediatr Pulmonol 2003;36:427-432. [PubMed]
Khan TZ, Wagener JS, Bost T, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995; 151: 1075-1082. [PubMed]
King M, Dasgupta B, Tomkiewicz RP, et al. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with rhDNase 1. Am J Respir Crit Care Med 1997; 156: 173-177. [PubMed]
Kirchner KK, Wagener JS, Khan TZ, et al. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med 1996; 154: 1426-1429. [PubMed]
Konstan MW, Wagener JS, Pasta DJ. Pulmozyme (Dornase alfa) use is associated with a slower rate of lung function decline in patients with cystic fibrosis. Pediatr Pulmonol 2006; Suppl 29: 337.
McCoy K. Hamilton S. Johnson C. Effects of 12-week administration of dornase alfa in patients with advanced cystic fibrosis lung disease. Pulmozyme Study Group. Chest 1996; 110: 889-895. [PubMed]
Milla CE. Long term effects of aerolised rhDNase on pulmonary disease progression in patients with cystic fibrosis. Thorax 1998; 53: 1014-1017. [PubMed]
Nasr SZ, Kuhns LR, Brown RW, et al. Use of computerized tomography and chest x-rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than 5 years: a preliminary study. Pediatr Pulmonol 2001; 31: 377-382. [PubMed]
Paul K, Rietschel E, Ballman M, et al. Effect of treatment with Dornase Alpha on airway inflammation in patients with cystic fibrosis. Am J Crit Care Med 2004; 169: 719-725. [PubMed]
Potter J, Mathews LW, Lemm J, et al. The composition of pulmonary secretions from patients with and without cystic fibrosis. Am J Dis Child 1960; 100: 493-5.
Quan JM, Tiddens HA, Sy JP, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr 2001;139:813-20. [PubMed]
Ranasinha C, Assoufi B, Shak S, et al. Efficacy and safety of short term administration of aerosolised recombinant human DNase 1 in adults with stable stage cystic fibrosis. Lancet 1993; 342: 199-202. [PubMed]
Ratnalingham R, Vasudev N, Watson A, et al. Benefit from Pulmozyme® use in an adult Cf Unit 1994-2000. J Cyst Fibros 2001; Abstract Book; p81.
Rittie JL, Turck D, Roussey M, et al. Longterm study of rhDNase in CF patients: a four year French multicentre study. Pediatr Pulmonol 1999; Suppl 19: 284.
Raskin P. Bronchospasm after inhalation of pancreatic dornase. Am Rev Resp Dis 1968; 98: 697-698. [PubMed]
Ratjen F, Paul K, van Koningsbruggen S, et al. DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: influence of treatment with dornase alfa. Pediatr Pulmonol 2005; 39: 1-4. [PubMed]
Roos-Liegmann B, Schmidt H, Krackhardt B,et al. Recombinant human deoxyribonuclease 1 inhalation herapy in CF patients improves lung function but does not prevent progression of lung destruction. Pediatr Pulmonol 1999; Suppl 19: 278.
Salomon A, Hercuhfus JA, Segal MS. Aerosols of pancreatic dornase in bronchopulmonary disease. Annals of Allergy. 1954 (Jan/Feb):71-79. [PubMed]
Sanders NN, Franckx H, De Boeck K, et al. Role of magnesium in the failure of rhDNase therapy in patients with cystic fibrosis. Thorax 2006; 61: 962-968. [PubMed]
Spier R, Witebsky E, Paine JR. Aerosolized Pancreatic Dornase and Antibiotics in pulmonary infections. JAMA 1961; Dec: 878-886. [PubMed]
Shah PL, Scott SF, Geddes DM, et al. Two years experience with recombinant human DNase I in the treatment of pulmonary disease in cystic fibrosis. Respiratory Medicine 1995; 89(7):499-502. [PubMed]
Shah PL, Conway S, Scott SF, et al. A case-controlled study with dornase alfa to evaluate impact on disease progression over a 4-year period. Respiration. 68(2):160-4, 2001. [PubMed]
Shah PL, Bush A, Canny GJ, et al. Recombinant human DNase I in cystic fibrosis patients with severe pulmonary disease: a short-term, double-blind study followed by six months open-label treatment. Eur Respir J 1995; 8: 954-958. [PubMed]
Shak S, Capon DJ, Hellmiss R, et al. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci USA.1990; 87: 9188-9192. [PubMed]
Slattery DM, Waltz DA, Denham B, et al. Bronchoscopically administered recombinant human DNase for lobar atelectasis in cystic fibrosis. Pediatr Pulmonol 2001; 31: 383-8. [PubMed]
Suri R, Marshall LJ, Wallis C, et al. Effects of recombinant human DNase and hypertonic saline on airway inflammation in children with cystic fibrosis. Am J Respir Crit Care Med. 2002; 166: 352-355. [PubMed]
Touleimat BA, Conoscenti CS, Fine JM. Recombinant human DNase in management of lobar atelectasis due to retained secretions. Thorax 1995; 50: 1319-1321. [PubMed]
Van der Giessen LJ, de Jongste JC, Gosselink R, et al. RhDNase before airway clearance therapy improves airway patency in children with CF. Pediatr Pulmonol 2007; 42: 624-630. [PubMed]
Wagener JS, Rock MJ, Mc Cubbin MM, et al. Aerosol delivery and safety of recombinant human deoxyribonuclease in young children with cystic fibrosis: a bronchoscopic study. Pulmozyme Pediatric Broncoscopy Study Group. J Pediatr. 1998; 133: 486-491. [PubMed]
Zach MS. The role of recombinant human DNase in the treatment of patients with cystic fibrosis: many promises, more problems. Thorax. 1996; 51: 750-755. [PubMed]
