Nasal potential difference measurements
Introduction
Nasal potential difference (PD) measurements are used to assess the voltage across nasal epithelium, which correlates with the transport of sodium and chloride across cell membranes. Nasal PD was first demonstrated to be abnormal in Cystic Fibrosis (CF) in 1981 and the technique has since been used to increase our understanding of this condition (Knowles et al, 1981; Knowles et al, 1982; Hay & Geddes, 1985; Alton et al, 1987). It is now established as an important diagnostic tool and more recently has been used to assess the effectiveness of new treatments such as gene therapy (Alton et al, 1990; Caplen et al, 1995; Knowles et al, 1995; Hay et al, 1995; Zabner et al, 1996; Gill et al, 1997; Porteous et al, 1997; Noone et al, 2000; Hyde et al, 2000).
To assist in the evaluation of difficult cases a complete bioelectrical profile should be carried out. This includes basal potental difference, response to perfusion with amiloride and to chloride free solution in conjunction with isoprenaline. Similar electrophysiological tests can be performed across small bowel mucosa using an ussing chamber.
Method
Because epithelia are “electrically tight” (i.e. they have a resistance across them), the active secretion or absorption of charged salts such as sodium (Na+) and chloride (Cl-) ions causes a potential difference or voltage (Gatzy, 1967). Different epithelia have different ion transport characteristics and the magnitude of PD varies, depending on the site of measurement. Potential difference can be measured by using a high impedance voltmeter between two electrodes, one on the inside and one on the outside of the epithelia. The electrode on the outside (the exploring electrode) rests against the surface of the target epithelium. The internal electrode (the reference electrode) can be in any internal compartment of the body, although generally the subcutaneous tissue of the forearm is used. On paper this appears straight forward, however in practice, some skill and a good deal of experience are required to achieve accuracy and repeatability with this method.
The nasal cavity is accessible which makes it a good site to examine the ion transport characteristics of airway epithelia. Less than a centimetre into the nose the squamous (“skin type”) epithelium becomes ciliated pseudocolumnar epithelium, characteristic of the proximal airways (Carson et al, 1981). By employing the nasal PD measurement, Knowles and his colleagues were able to demonstrate that Na+ absorption was the primary ion transport activity in normal airway epithelia (Knowles et al, 1981). The resulting PD is negative when viewed from the outside and in people without CF would be of the magnitude of -15 to -25 mV; dependent to some degree on the position in the nose that the PD is measured.
Amiloride: Amiloride, a drug known to block Na+ absorption, causes a reduction in PD magnitude (i.e., the PD becomes less negative). However, there is nearly always some residual negative PD, presumably related to anion secretion (HCO3- or Cl-).
Chloride free solution: When the epithelium is bathed with a solution with low or zero levels of Cl- ions then a potential environment for Cl- secretion is generated. In non-CF epithelia, this results in a rapid and often large hyperpolarisation of the PD, which in the presence of amiloride is assumed to be Cl- secretion.
Isoprenaline: This increase in negative PD can be further enhanced by the addition of agents known to augment Cl- secretion, such as the beta agonist, isoprenaline or ATP (Middleton et al, 1994; Knowles et al, 1995). Isoprenaline increases intracellular cyclic AMP that activates Cl- secretion through the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) chloride channel, the putative protein in Cystic Fibrosis.
ATP: The purine ATP activates chloride secretion through alternative “non-CFTR” channels that have yet to be fully characterised (Knowles et al, 1991).
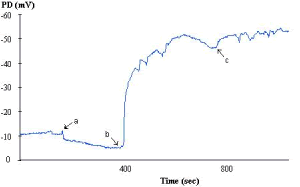
Figure 1. Nasal PD profile from a non-CF subject. The baseline PD is rather low, even for normal, (at around -10mV) and so is the subsequent response to amiloride (perfusion started at point a). The response to perfusion with a solution low in chloride ions is large (point b). This specific response is the best discriminator between non-CF and CF profiles (see later figure). The final perfusion change is the addition of ATP (point c). This is the most variable response in normal epithelia. Some subjects have a consistently large (though transient response). In most cases the response is small. This contrasts to CF subjects, in whom the response to ATP is generally large (although again transient).
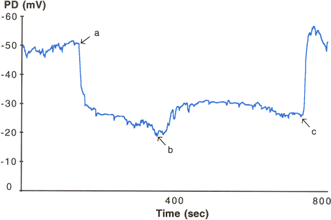
Figure 2. Nasal PD profile from a CF subject. The baseline PD is high (at around -50mV) and the subsequent response to amiloride is large (perfusion started at point a, nearly 30 mV). The response to perfusion with a solution low in chloride ions (point b) is small and demonstrates the “falling away” that is characteristic of CF. The final perfusion change is the addition of ATP (point c). In CF this generally (although not always) leads to a large response, which after an initial peak falls away. Many have advocated adding ATP (or another purine) at the end of the PD measurement as an indicator of epithelial integrity. For example, the finding of a normal or low baseline and a small response to a low chloride solution may be related to epithelial inflammation (for example, if the subject has a cold). However, if there is a subsequent large response to perfusion with ATP this would argue against that and make the result more worrying.
Healthy and CF nasal PD measurements
The change in PD with the perfusion of different solutions is demonstrated. It can be seen, therefore, that by employing nasal PD protocols with perfusion of different solutions and drugs, different aspects of the nasal ion transport characteristics can be examined. In CF, this ion transport profile is abnormal and the nasal PD measurement has a number of features that characterise CF from non-CF.
The CF nasal PD profile
Much work has been published characterising the CF nasal PD profile in adults and some work is beginning to emerge on children (Munck et al, 1999; Southern et al, 1999). People with CF have a much larger baseline PD (i.e., more negative) than non-CF. They also have an increased reduction in the magnitude of PD following perfusion with amiloride. This suggests that increased Na+ absorption is a primary defect in the CF airway. Other work on ex vivo models (animal and tissue culture) has confirmed Na+ hyperabsorption in CF airway epithelia. Recently this has been demonstrated to be an important factor in the pathophysiology associated with CF airways. The large baseline PD and amiloride response in CF are impressive, however the most consistent abnormality in the CF nasal PD profile is the response of the PD to perfusion with a zero or low chloride solution. In contrast to the large sustained response in non-CF epithelia there is an initial small response in CF epithelia, which then returns to previous levels. There is some evidence to suggest that the addition of a beta-agonist, such as isoprenaline, increases the differentiation between CF and non-CF (Middleton et al, 1994; Knowles et al, 1995). The addition of ATP or another purine to the low chloride solution does lead to a large, if transient hyperpolarisation in CF tissues, presumably through non-CFTR mediated chloride secretion. The initial recognition of this response resulted in the examination of purinergic agents as potential therapies for CF (Olivier et al, 1996).
In summary, it can be seen that by employing a perfusion protocol both sodium and chloride epithelial transport can be examined, both of which are characteristically abnormal in CF.
Some comments on methodology
In contrast to say the sweat test, there is no internationally accepted method for measuring nasal PD. Papers have described perfusion protocols but the practicalities of the test vary considerably between different groups. Examples are; type and position of reference electrode, single or double lumen catheter, the temperature of perfusates, Silver Chloride (AgCl) or calomel electrodes, which part of the nasal epithelia to measure, what specific perfusion solutions to use, the dose of amiloride etc. Each investigator tends to remain loyal to their method (or modify another). This is probably not a significant problem as long as methodology is stated clearly in papers, in order that data can be critically evaluated. One aspect that is significant is the site of measurement. In his initial work Knowles measured PD along the inferior aspect of the inferior turbinate, as preliminary studies suggested this to be the area of highest PD (Knowles et al, 1981). This is an area rich in ciliated epithelial cells, in contrast to the anterior portion or tip of the turbinate where the epithelia is more squamous in nature and the PD lower (Carson et al, 1981). Many workers have followed this example. However, others, predominately in Europe have employed measurements along the floor of the nasal cavity. Here the baseline PD is significantly less, although data suggest that low chloride responses are equally large in non-CF subjects. Certainly measurements along the floor are technically much more straightforward and therefore possibly more repeatable. There is significant variability in the PD measurement (both intra and inter personal) and care must be taken not to over interpret results, particularly with small differences between groups. What makes nasal PD a useful test is the extent of difference between CF and non-CF. Various parts of the nasal PD profile are characteristically abnormal. Analysing the whole of the PD profile is therefore of more value than individual components. It is important to remember that the magnitude of baseline PD can be reduced with inflammation. Certain drugs and exercise also affect the baseline PD (Alsuwaidan et al, 1994; Peckham et al, 1995; Middleton et al, 1996)

Figure 3. Practical procedure
PD measurement in children
Measurements on children and infants have previously employed adult methods (Gowen et al, 1986; Sauder et al, 1987; Wilson et al, 1998). However groups are now developing techniques that are far better suited to the paediatric population (Munck et al, 1999). These methods use smaller, often single lumen catheters with much lower perfusion rates (0.2 ml min-1 compared to up to 5 ml min-1 with the adult method Shorter protocol times have also been advocated making the test tolerable for even the smallest child (although obviously some degree of cooperation is necessary and therefore measurements in children less than five remain difficult). Perfusion measurements are possible in newly born infants. Obviously this opens up the potential for using PD as a diagnostic test. This is important as the diagnosis of CF is often difficult to make or refute in babies because of the problems in collecting enough sweat and because mutational analysis is often negative.
Summary
The nasal PD measurement provides a tool for examining the fundamental defect in CF, namely the abnormality of salt transport across the airway epithelia. As such nasal PD has increased our understanding of CF and allowed correlations between disease severity and ion transport. In addition this measurement has provided a valuable diagnostic tool and efficacy measure for new therapies.
References
Alsuwaidan S, Li Wan Po A, Morrison G, et al. Effect of exercise on the nasal transmucosal potential difference in patients with cystic fibrosis and normal subjects. Thorax 1994;49:1249-50. [PubMed]
Alton EW, Hay JG, Munro C, Geddes DM. Measurement of nasal potential difference in adult cystic fibrosis, Young’s syndrome, and bronchiectasis. Thorax 1987;42:815-7. [PubMed]
Alton EW, Currie D, Logan-Sinclair R, et al. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J 1990;3:922-6. [PubMed]
Caplen NJ, Alton EW, Middleton PG, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med 1995;1:39-46. [PubMed]
Carson JL, Collier AM, Knowles MR, et al. Morphometric aspects of ciliary distribution and ciliogenesis in human nasal epithelium. Proc Natl Acad Sci U S A 1981;78:6996-9. [PubMed]
Gatzy JT. Bioelectric properties of the isolated amphibian lung. Am J Physiol 1967;213:425-31. [PubMed]
Gill DR, Southern KW, Mofford KA, et al. A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997;4:199-209. [PubMed]
Gowen CW, Lawson EE, Gingras-Leatherman J, et al. Increased nasal potential difference and amiloride sensitivity in neonates with cystic fibrosis. J Pediatr 1986;108:517-21. [PubMed]
Hay JG, Geddes DM. Transepithelial potential difference in cystic fibrosis. Thorax 1985;40:493-496. [PubMed]
Hay JG, McElvaney NG, Herena J, Crystal RG. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum Gene Ther. 1995;6:1487-96. [PubMed]
Hyde SC, Southern KW, Gileadi U, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis.Gene Ther 2000;7:1156-65. [PubMed]
Knowles MR, Gatzy JT, Boucher RC. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Eng J Med 1981;305:1489-95. [PubMed]
Knowles MR, Carson JL, Collier AM, et al. Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am Rev Respir Dis 1981;124:484-90. [PubMed]
Knowles MR, Buntin WH, Bromberg PA, et al. Measurements of transepithelial electric potential differences in the trachea and bronchi of human subjects in vivo. Am Rev Respir Dis 1982;126:108-12. [PubMed]
Knowles MR, Hohneker KW, Zhou Z, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med 1995;333:823-31. [PubMed]
Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther 1995;6:445-455. [PubMed]
Knowles MR, Clarke LL, Boucher RC. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med 1991;325:533. [PubMed]
Middleton PG, Geddes DM, Alton EW. Protocols for in vivo measurement of the ion transport defects in cystic fibrosis nasal epithelium. Eur Respir J 1994;7:2050-2056. [PubMed]
Middleton PG, Geddes DM, Alton EW. Trimethoprim and tetracycline inhibit airway epithelial sodium absorption. Am J Respir Crit Care Med 1996;154:18-23. [PubMed]
Munck A, Gerardin M, Godard A, et al. Nasal transepithelial potential difference in healthy young children. Ped Pulmonol 1999;suppl 19;215 (abstract 180)
Noone PG, Hohneker KW, Zhou Z, et al. Safety and biological efficacy of a lipid-CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis. Mol Ther 2000;1:105-14. [PubMed]
Olivier KN, Bennett WD, Hohneker KW, et al. Acute safety and effects on mucociliary clearance of aerosolized uridine 5′-triphosphate +/- amiloride in normal human adults. Am J Respir Crit Care Med 1996;154:217-23. [PubMed]
Peckham DG, Conn A, Chotai C, et al. Effect of oral digoxin, topical ouabain and salbutamol on transepithelial nasal potential difference in patients with cystic fibrosis. Clin Sci (Colch) 1995;89:277-84. [PubMed]
Porteous DJ, Dorin JR, McLachlan G, et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transferto the nasal epithelium of patients with cystic fibrosis. Gene Ther 1997;4:210-8. [PubMed]
Sauder RA, Chesrown SE, Loughlin GM. Clinical application of transepithelial potential difference measurements in cystic fibrosis. J Pediatr 1987;111:353-8. [PubMed]
Southern KW, Bosworth DG, Kazachkova IA, et al. Nasal potential difference measurement for the diagnosis of Cystic Fibrosis in neonates, infants and children Ped Pulmonol 1999;suppl 19;215 (abstract 181).
Wilson DC, Ellis L, Zielenski J, et al. Uncertainty in the diagnosis of cystic fibrosis: possible role of in vivo nasal potential difference measurements. J Pediatr 1998;132:596-599. [PubMed]
Zabner J, Ramsey BW, Meeker DP, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest 1996;97:1504-11. [PubMed]
