SECTION TWO – GENERAL MODULATOR REFERENCES FROM 2020
THERE MAY BE SOME DUPLICATES AS THEY WILL HAVE BEEN FIRST PICKED UP WHEN PUBLISHED ON LINE
2020 2020 2020
B L Aalbers , R W Hofland , I Bronsveld , K M de Winter-de Groot , H G M Arets , A C de Kiviet , M M M van Oirschot-van de Ven, M A Kruijswijk, S Schotman, S Michel, C K van der Ent, H G M Heijerman. Females With Cystic Fibrosis Have a Larger Decrease in Sweat Chloride in Response to lumacaftor/ivacaftor Compared to Males. J Cyst Fibros 2020 May 21;S1569-1993(20)30135-1. [Pubmed]
A study to explore which patient-related factors influence sweat test response to CFTR modulators, as well as examining the correlation between the sweat chloride response and ppFEV1 or BMI response, using systematically collected real-life clinical data.
160 CF patients were identified who had used lumacaftor/ivacaftor (ORKAMBI) for at least six months. Of these patients, age, sweat chloride levels, ppFEV1 weight and BMI at the start of treatment and after 6 months were collected retrospectively. Pearson and Spearman tests were performed to assess correlations.
Results: Females compared to males in this group showed a larger response in sweat chloride (mean difference 10.6 mmol/l, 95% CI: 5.7-15.4) and BMI (mean difference 0.27 kg/m2, 95% CI: 0.01-0.54). A modest but significant correlation was found between patient weight and sweat chloride response (Pearson R = 0.244, p = 0.001), which diminished upon correction for the other factors. The correlation between sex and sweat chloride response remained; R = 0.253, p = 0.001. Sweat chloride response did not correlate with ppFEV1 change or BMI change at 6 months after start of therapy.
The authors concluded that the Sweat chloride response is larger in females compared to males, which also explains the negative correlation of weight with the response in sweat chloride concentration after start of lumacaftor/ivacaftor (ORKAMBI). Sweat chloride response does not correlate with the responses in ppFEV1and BMI. This information may help the interpretation of sweat test results acquired for the follow up and evaluation of CFTR modulating treatments and warrants further investigation into the underlying mechanisms of sex differences in response to CFTR modulators.
Dr B L Aalbers is in the Department of Pulmonology, University Medical Center Utrecht, the Netherlands.
Conclusions: Treatment with ivacaftor plus best supportive care versus best supportive care alone is not cost-effective at or near commonly accepted WTP thresholds.
Keel Wherry is the Director, Health Economics and Outcomes Research at Medtronics, Minneapolis, MN, USA.
B L Aalbers , K M de Winter-de Groot , H G M Arets , R W Hofland , A C de Kiviet , M M M van Oirschot-van de Ven , M A Kruijswijk , S Schotman , S Michel , C K van der Ent , H G M Heijerman. Clinical Effect of lumacaftor/ivacaftor in F508del Homozygous CF Patients With FEV 1 ≥ 90% Predicted at Baseline. J Cyst Fibros. 2020 Jul;19(4):654-658.doi: 10.1016/j.jcf.2019.12.015. Epub 2020 Jan 7. [Pubmed]
Objective: The first available CFTR modulator combination for homozygous F508del patients, lumacaftor/ivacaftor (ORKAMBI) , has not been tested in patients with percentage predicted (pp)FEV1 > 90 in the phase III trials. The objective of this study is to share real life experience about treatment results in this group. In this retrospective observational study, patients aged 6 years or older starting on lumacaftor/ivacaftor in standard care were in strict follow up. For these patients, data were obtained about FEV1, BMI, CFQ-R and sweat chloride before start and after 6 months of treatment, and data about FEV1 and BMI were recorded every 3 months. Exacerbations were recorded continuously.
Results: They identified 40 patients with a ppFEV1 ≥ 90 at the start of lumacaftor/ivacaftor who had been in follow up for at least 12 months. After 12 months, ppFEV1 was unchanged, whereas mean absolute change in BMI was +0.88 (p = 0.001) with a mean change in SDS for BMI of +0.26 (p = 0.014). Mean CFQ-R overall score at 6 months improved by 2.6% (p = 0.004) and mean decrease in sweat chloride was -27.3 mEq/L (p = 0.000). Exacerbation rate declined from 1.03 to 0.53/person/year (p = 0.003). One patient discontinued treatment in the first 12 months because of progression of CFRLD, two paused treatment but resumed later.
Conclusion: Homozygous F508del patients starting lumacaftor/ivacaftor at ppFEV1 ≥ 90 improved significantly in nutritional status, sweat chloride levels and exacerbation rate, but did not respond in ppFEV1. Treatment is well tolerated in this patient group. These effects make it worth considering to treat this group of patients with lumacaftor/ivacaftor.
Dr B L Aalbers is in the Department of Pulmonology, University Medical Center Utrecht, the Netherlands.
Albright JC, Houck AP, Pettit RS. Effects of CFTR modulators on pharmacokinetics of tobramycin during acute pulmonary exacerbations in the pediatric cystic fibrosis population [published online ahead of print, 2020 Jun 22]. Pediatr Pulmonol. 2020;10.1002/ppul.24917.
Individuals with cystic fibrosis (CF) require higher dosages of aminoglycosides due to an increased volume of distribution (Vd ) and clearance. Optimal dosing of aminoglycosides in the CF population is essential as repeated exposure to aminoglycosides during acute pulmonary exacerbations increases risk of nephrotoxicity and ototoxicity. To date, no studies have evaluated whether chronic CFTR modulator therapy affects pharmacokinetics of aminoglycoside antibiotics in CF patients. The objective of this study was to determine if the addition of a CFTR modulator affects elimination rate (Ke ) for intravenously administered tobramycin in the pediatric CF population
This retrospective study included patients aged 2 to 18 years with CF receiving chronic therapy with a CFTR modulator. Patients included had an admission both pre- and post-chronic CFTR modulator therapy during which they received therapy with IV tobramycin.
The pharmacokinetic parameters of intravenously administered tobramycin during admission for acute pulmonary exacerbation do not appear to change significantly after initiating chronic therapy with a CFTR modulator. Empiric dose adjustments for patients on CFTR modulators are not recommended. This article is protected by copyright. All rights reserved.
Dr Jared C Albright is at the Riley Hospital for Children at IU Health, Indianapolis.
Allobawi R, Ghelani DP, Schneider-Futschik EK. Metabolomic Description of Ivacaftor Elevating Polymyxin B Mediated Antibacterial Activity in Cystic Fibrosis Pseudomonas aeruginosa. ACS Pharmacol Transl Sci. 2020;3(3):433-443. Published 2020 Apr 27. doi:10.1021/acsptsci.0c00030 [Pubmed]

Dr Elena Schneider- Futschik
We have demonstrated that ivacaftor displays synergistic antibacterial activity in combination with polymyxin B against polymyxin-resistant Pseudomonas aeruginosa that commonly colonizes the lungs of people with cystic fibrosis (CF). However, the underlying mechanism(s) remain unclear. In the present study, we employed untargeted metabolomics to investigate synergistic killing mechanism of polymyxin B in combination with ivacaftor against a polymyxin-susceptible P. aeruginosa FADDI-PA111 (polymyxin B MIC = 2 mg/L) and a polymyxin-resistant CF P. aeruginosaFADDI-PA006 (polymyxin B MIC = 8 mg/L). Metabolites were extracted at 3 h after treatments with polymyxin B alone (2 μg/mL for FADDI-PA111 and 4 μg/mL FADDI-PA006 P. aeruginosa), ivacaftor alone (8 μg/mL), and in combination. Polymyxin B monotherapy induced significant perturbations in the glycerophospholipid and fatty acid metabolism pathways against FADDI-PA111 and to a lesser extent in FADDI-PA006. In both strains, treatment with ivacaftor alone induced more pronounced perturbations in glycerophospholipid and fatty acid metabolism pathways than that with polymyxin B alone. This highlights the unique antimicrobial mode of action of ivacaftor. Pathway analysis revealed that in combination treatment, polymyxin B mediated killing is elevated by ivacaftor, largely due to the inhibition of cell envelope biogenesis via suppression of key membrane lipid metabolites (e.g., sn-glycerol 3-phosphate and sn-glycero-3-phosphoethanolamine) as well as perturbations in peptidoglycan and lipopolysaccharide biosynthesis. Furthermore, significant perturbations in the levels of amino sugars and nucleotide sugars, glycolysis, the tricarboxylic acid cycle, and pyrimidine ribonucleotide biogenesis were observed with the combination treatment. These findings provide novel mechanistic information on the synergistic antibacterial activity of polymyxin-ivacaftor combination.
Department of Pharmacology & Therapeutics, School of Biomedical Sciences, Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Parkville, Victoria 3010, Australia
Margarida D Amaral, Margarida C Quaresma, Ines Pankonien.What Role Does CFTR Play in Development, Differentiation, Regeneration and Cancer?Int J Mol Sci 2020 Apr 29;21(9):E3133.doi: 10.3390/ijms21093133. [Pubmed]

Margarida Quaresma

Margarida Amaral
One of the key features associated with the substantial increase in life expectancy for individuals with CF is an elevated predisposition to cancer, firmly established by recent studies involving large cohorts. With the recent advances in cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies and the increased long-term survival rate of individuals with cystic fibrosis (CF), this is a novel challenge emerging at the forefront of this disease. However, the mechanisms linking dysfunctional CFTR to carcinogenesis have yet to be unravelled. Clues to this challenging open question emerge from key findings in an increasing number of studies showing that CFTR plays a role in fundamental cellular processes such as foetal development, epithelial differentiation/polarization, and regeneration, as well as in epithelial-mesenchymal transition (EMT).
Here, we provide state-of-the-art descriptions on the moonlight roles of CFTR in these processes, highlighting how they can contribute to novel therapeutic strategies. However, such roles are still largely unknown, so we need rapid progress in the elucidation of the underlying mechanisms to find the answers and thus tailor the most appropriate therapeutic approaches.
From the BioISI-Biosystems and Integrative Sciences Institute, Faculty of Sciences, University of Lisboa, Portugal.
– A very interesting detailed and timely review of this important subject from Margarida Amaral’s laboratory.
Aoyama BC, Mogayzel PJ. Ivacaftor for the treatment of cystic fibrosis in children under six years of age.
Expert Rev Respir Med. 2020 Mar 17:1-11. doi: 10.1080/17476348.2020.1741352. [Epub ahead of print] [Pubmed]
The authors reviewed the sentinel studies that lead to the approval of the use of ivacaftor in people with CF age six months and older with at least one CFTR gene mutation that is responsive to ivacaftor based on clinical trial and/or in vitro data. Children with CF have the greatest potential to benefit from CFTR modulator therapy when it is initiated prior to the development of permanent damage; however, challenges remain regarding use of ivacaftor in the youngest pediatric population. Ivacaftor is safe and effective CFTR modulator that can be prescribed in children over six months of age with at least one CFTR gene mutation that is responsive to ivacaftor.
From the Eudowood Division of Pediatric Respiratory Sciences, Johns Hopkins University School of Medicine, Baltimore,
Elizabeth Baker , William T Harris, Steven M Rowe, Sarah B Rutland, Gabriela R Oates. Tobacco smoke exposure limits the therapeutic benefit of tezacaftor/ivacaftor in pediatric patients with cystic fibrosis. J Cyst Fibros 2020 Oct 3;S1569-1993(20)30870-5.doi: 10.1016/j.jcf.2020.09.011.Online ahead of print.[Pubmed]

Elizabeth Baker
Objectives: Tobacco smoke exposure reduces CFTR functional expression in vitro and contributes to acquired CFTR dysfunction. We investigated whether it also inhibits the clinical benefit of CFTR modulators, focusing on tezacaftor/ivacaftor, approved in February 2018 for individuals with CF age ≥12 years.
Methods: A retrospective longitudinal analysis of encounter-based data from the CF Foundation Patient Registry (2016-2018) compared the slope of change in lung function (GLI FEV1% predicted) before and after tezacaftor/ivacaftor initiation in smoke-exposed vs unexposed age-eligible pediatric patients. Tobacco smoke exposure (Ever/Never) was determined from caregiver self-report. Statistical analyses used hierarchical linear mixed modeling and fixed effects regression modeling.
Results: The sample included 6,653 individuals with a total of 105,539 person-period observations. Tezacaftor/ivacaftor was prescribed to 19% (1,251) of individuals, mean age 17 years, mean baseline ppFEV1 83%, 28% smoke-exposed. Tezacaftor/ivacaftor users who were smoke-exposed had a lower baseline ppFEV1 and experienced a greater lung function decline. Over two years, the difference in ppFEV1 by smoke exposure among tezacaftor/ivacaftor users increased by 1.2% (7.6% to 8.8%, p<0.001). In both mixed effects and fixed effects regression models, tezacaftor/ivacaftor use was associated with improved ppFEV1 among unexposed individuals (1.2% and 1.7%, respectively; p<0.001 for both) but provided no benefit among smoke-exposed counterparts (0.3%, p = 0.5 and 0.6%, p = 0.07, respectively).
Conclusion: Tobacco smoke exposure nullifies the therapeutic benefit of tezacaftor/ivacaftor among individuals with CF aged 12-20 years old. To maximize the therapeutic opportunity of CFTR modulators, every effort must be taken to eliminate smoke exposure in CF.
Dr Elizabeth Baker is in the department of sociology at University of Alabama at Birmingham, Birmingham, AL, USA
I M Balfour-Lynn , J A King. CFTR Modulator Therapies – Effect on Life Expectancy in People With Cystic Fibrosis. Paediatr Respir Rev 2020 May 26;S1526-0542(20)30081-6. doi: 10.1016/j.prrv.2020.05.002. Online ahead of print. [Pubmed]

Ian Balfour-Lynn
CFTR modulators have dramatically changed the clinical course of CF in those fortunate enough to receive them. Inevitably, randomised controlled trials during the development of these drugs are too short to use mortality as an outcome. Evidence for their effect on life expectancy are best gained from real world registry studies specifically looking at mortality, but these are only available for ivacaftor to date. Therefore, indirect evidence must be obtained by looking at outcomes known to affect mortality and seeing the effect of these drugs on those outcomes
Dr Ian Balfour-Lynn is a consultant paediatrician at the Royal Brompton Hospital, London
Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, Burgel PR, Tullis E, Castaños C, Castellani C, Byrnes CA, Cathcart F, Chotirmall SH, Cosgriff R, Eichler I, Fajac I, Goss CH, Drevinek P, Farrell PM, Gravelle AM, Havermans T, Mayer-Hamblett N, Kashirskaya N, Kerem E, Mathew JL, McKone EF, Naehrlich L, Nasr SZ, Oates GR, O’Neill C, Pypops U, Raraigh KS, Rowe SM, Southern KW, Sivam S, Stephenson AL, Zampoli M, Ratjen F. The Future of Cystic Fibrosis Care: A Global Perspective. Lancet Respir Med Jan 2020;8(1): 65-124 [Pubmed]

Felix Ratjen

Scott Bell
The past six decades have seen remarkable improvements in health outcomes for people with cystic fibrosis, which was once a fatal disease of infants and young children. However, although life expectancy for people with cystic fibrosis has increased substantially, the disease continues to limit survival and quality of life, and results in a large burden of care for people with cystic fibrosis and their families. Furthermore, epidemiological studies in the past two decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognised in many regions of the world. The Lancet Respiratory Medicine Commission on the future of cystic fibrosis care was established at a time of great change in the clinical care of people with the disease, with a growing population of adult patients, widespread genetic testing supporting the diagnosis of cystic fibrosis, and the development of therapies targeting defects in the cystic fibrosis transmembrane conductance regulator (CFTR), which are likely to affect the natural trajectory of the disease. The aim of the Commission was to bring to the attention of patients, health-care professionals, researchers, funders, service providers, and policy makers the various challenges associated with the changing landscape of cystic fibrosis care and the opportunities available for progress, providing a blueprint for the future of cystic fibrosis care. The discovery of the CFTR gene in the late 1980s triggered a surge of basic research that enhanced understanding of the pathophysiology and the genotype-phenotype relationships of this clinically variable disease. Until recently, available treatments could only control symptoms and restrict the complications of cystic fibrosis, but advances in CFTR modulator therapies to address the basic defect of cystic fibrosis have been remarkable and the field is evolving rapidly. However, CFTR modulators approved for use to date are highly expensive, which has prompted questions about the affordability of new treatments and served to emphasise the considerable gap in health outcomes for patients with cystic fibrosis between high-income countries, and low-income and middle-income countries (LMICs). Advances in clinical care have been multifaceted and include earlier diagnosis through the implementation of newborn screening programmes, formalised airway clearance therapy, and reduced malnutrition through the use of effective pancreatic enzyme replacement and a high-energy, high-protein diet. Centre-based care has become the norm in high-income countries, allowing patients to benefit from the skills of expert members of multidisciplinary teams. Pharmacological interventions to address respiratory manifestations now include drugs that target airway mucus and airway surface liquid hydration, and antimicrobial therapies such as antibiotic eradication treatment in early-stage infections and protocols for maintenance therapy of chronic infections. Despite the recent breakthrough with CFTR modulators for cystic fibrosis, the development of novel mucolytic, anti-inflammatory, and anti-infective therapies is likely to remain important, especially for patients with more advanced stages of lung disease. As the median age of patients with cystic fibrosis increases, with a rapid increase in the population of adults living with the disease, complications of cystic fibrosis are becoming increasingly common. Steps need to be taken to ensure that enough highly qualified professionals are present in cystic fibrosis centres to meet the needs of ageing patients, and new technologies need to be adopted to support communication between patients and health-care providers. In considering the future of cystic fibrosis care, the Commission focused on five key areas, which are discussed in this report: the changing epidemiology of cystic fibrosis (section 1); future challenges of clinical care and its delivery (section 2); the building of cystic fibrosis care globally (section 3); novel therapeutics (section 4); and patient engagement (section 5). In panel 1, we summarise key messages of the Commission. The challenges faced by all stakeholders in building and developing cystic fibrosis care globally are substantial, but many opportunities exist for improved care and health outcomes for patients in countries with established cystic fibrosis care programmes, and in LMICs where integrated multidisciplinary care is not available, and resources are lacking at present. A concerted effort is needed to ensure that all patients with cystic fibrosis have access to high-quality health care in the future.
Scott Bell and Felix Ratjen coordinated the Commision and reviewed and edited all sections of the paper.
Prof. Scott Bell of the Department of Thoracic Medicine, The Prince Charles Hospital, Brisbane, QLD, Australia; QIMR Berghofer Medical Research Institute, Brisbane, QLD, Australia. Previous editor of the JCF.
Prof. Felix Ratjen of the Division of Respiratory Medicine, Department of Paediatrics Translational Research Programme, Hospital for Sick Children, University of Toronto, Toronto, Canada
-This really impressive article by 38 of the world’s experts with 522 references can be unreservedly recommended for anyone who wishes a detailed comprehensive readable review of the subject.
Bose SJ, Krainer G, Ng DRS, Schenkel M, Shishido H, Yoon JS, Haggie PM, Schlierf M, Sheppard DN, Skach WR. Towards next generation therapies for cystic fibrosis: Folding, function and pharmacology of CFTR. J Cyst Fibros. 2020 Jan 2. pii: S1569-1993(19)30989-0. doi: 10.1016/j.jcf.2019.12.009. [Epub ahead of print] [Pubmed]
The treatment of cystic fibrosis (CF) has been transformed by orally-bioavailable small molecule modulators of the cystic fibrosis transmembrane conductance regulator (CFTR), which restore function to CF mutants. However, CFTR modulators are not available to all people with CF and better modulators are required to prevent disease progression. Here, they review selectively recent advances in CFTR folding, function and pharmacology. They highlight ensemble and single-molecule studies of CFTR folding, which provide new insight into CFTR assembly, its perturbation by CF mutations and rescue by CFTR modulators. They discuss species-dependent differences in the action of the F508del-CFTR mutation on CFTR expression, stability and function, which might influence pharmacological studies of CFTR modulators in CF animal models. Finally, they illuminate the identification of combinations of two CFTR potentiators (termed co-potentiators), which restore therapeutically-relevant levels of CFTR activity to rare CF mutations. Thus, mechanistic studies of CFTR folding, function and pharmacology inform the development of highly effective CFTR modulators.
The first author of this multinational paper is S J Bose from the School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol BS8 1TD, UK
Pierre-Régis Burgel , Anne Munck , Isabelle Durieu , Raphaël Chiron , Laurent Mely , Anne Prevotat et al, French Cystic Fibrosis Reference Network Study Group. Real-Life Safety and Effectiveness of Lumacaftor-Ivacaftor in Patients With Cystic Fibrosis. Am J Respir Crit Care Med. 2020 Jan 15;201(2):188-197. doi: 10.1164/rccm.201906-1227OC. [Pubmed]

Pierre-Regis Burgel
To evaluate the safety and effectiveness of lumacaftor-ivacaftor in adolescents (≥12 yr) and adults (≥18 yr) in a real-life post approval setting. The study was conducted in the 47 CF reference centers in France. All patients who initiated lumacaftor-ivacaftor from January 1 to December 31, 2016, were eligible. Patients were evaluated for lumacaftor-ivacaftor safety and effectiveness over the first year of treatment following the French CF Learning Society’s recommendations.
Main findings – Among the 845 patients (292 adolescents and 553 adults) who initiated lumacaftor-ivacaftor, 18.2% (154 patients) discontinued treatment, often owing to respiratory (48.1%, 74 patients) or non-respiratory (27.9%, 43 patients) adverse events. In multivariable logistic regression, factors associated with increased rates of discontinuation included adult age group, percent predicted FEV1 (ppFEV1) less than 40%, and numbers of intravenous antibiotic courses during the year before lumacaftor-ivacaftor initiation. Patients with continuous exposure to lumacaftor-ivacaftor showed an absolute increase in ppFEV1 (+3.67%), an increase in body mass index (+0.73 kg/m2), and a decrease in intravenous antibiotic courses by 35%. Patients who discontinued treatment had significant decrease in ppFEV1, without improvement in body mass index or decrease in intravenous antibiotic courses
Dr Pierre-Régis Burgel is at the Faculty of Medicine, Paris Descartes CPSC.
Chevalier B, Hinzpeter A. The influence of CFTR complex alleles on precision therapy of cystic fibrosis. J Cyst Fibros. 2020 Mar;19 Suppl 1:S15-S18. doi: 10.1016/j.jcf.2019.12.008. Epub 2019 Dec 26.[Pubmed] CFTR is an extensively studied gene and multiple sequence variants have been identified, many of which still need to be defined as neutral or disease causing. Complex alleles are defined when at least two variants are identified on the same allele. Each pathogenic variant can affect distinct steps of the CFTR biogenesis. As CFTR modulators are being developed to alleviate specific defects, pathogenic variants need to be characterized to propose adequate treatments. Conversely, cis-variants can affect treatment response when defects are additive or if they alter the binding or efficacy of the modulator. Hence, complex alleles increase the complexity of CFTR variant classification and need to be assigned as neutral, disease causing or modulating treatment efficacy. This review was based on a symposium session presented at the 16th ECFS Basic Science Conference, Dubrovnik, Croatia, 27 to 30 March, 2019.
Both authors from INSERM U1151, Institut Necker Enfants Malades, Paris, France; Université Paris Descartes, Paris, France. Electronic address: benoit.chevalier@inserm.fr.
Claudio Costantini, Matteo Puccetti, Marilena Pariano, Giorgia Renga, Claudia Stincardini, Fiorella D’Onofrio, Marina M Bellet, Barbara Cellini, Stefano Giovagnoli, Luigina Romani. Selectively targeting key inflammatory pathways in cystic fibrosis. Eur J Med Chem 2020 Nov 15;206:112717.doi: 10.1016/j.ejmech.2020.112717.Epub 2020 Aug 9.[Pubmed]
The development of modulators targeting the basic defect of CFTR has represented a major breakthrough in CF therapy, but the impact on inflammation has remained enigmatic. The emerging scenario taking hold in the field points to inflammation as a major, somehow missed, therapeutic target for prevention of lung decline. Not surprisingly, the development of anti-inflammatory drugs is taking its share in the drug development pipeline. But the path is not straightforward and targeting inflammation should be balanced with the increased risk of infection. The strategy to restore the homeostatic regulation of inflammation to efficiently respond to infection while preventing lung damage needs to be based on identifying and targeting endogenous immunoregulatory pathways that are defective in CF.
The authors provide an overview of anti-inflammatory drugs currently approved or under investigation in CF patients, and present our recent studies on how the knowledge on defective immune pathways in CF may translate into innovative and selective anti-inflammatory therapeutics.Through the discovery of naturally occurring molecules or their synthetic mimics, this review emphasizes the critical importance of selectively targeting key inflammatory pathways to preserve immunocompetence in CF patients.
Claudio Costantini is a post-doctoral researcher in the Department of Experimental Medicine, University of Perugia, Perugia, 06132, Italy.
Cuevas-Ocaña S, Laselva O, Avolio J, Nenna R. The era of CFTR modulators: improvements made and remaining challenges Breathe (Sheff). 2020 Jun;16(2):200016. doi: 10.1183/20734735.0016-2020. [Pubmed] PMC article.

Sara Cuevas-Ocaña
The entry into the clinic of CFTR modulators such as TRIKAFTA has significantly improved life for 90% CF patients carrying one or two F508del mutations but challenges remain for rare CFTR mutations and the management of lung infections.
This last decade has created historical moments for CF, primarily driven by the development of CFTR modulators. First for patients with gating mutations who benefited from Kalydeco, then for those patients with one F508del copy who could benefit from Orkambi, and most recently, patients with at least one F508del copy who can benefit from Trikafta. Despite heterogeneity in patient response, the majority of CF patients will be greatly impacted by using a CFTR modulator therapy, thus changing the trajectory of their life. Furthermore, it remains to be determined whether the next generation of modulators will be effective for individuals bearing rare mutations that are Orkambi resistant. However, it should not be forgotten that there still remains 10% of the CF population who do not have a targeted CFTR modulator treatment. In addition, even with these novel drug therapies, managing infections will continue to be a challenge, thus the CF community will need to adapt the standards for an improving, but ageing CF population.
Dr Sara Cuevas-Ocaña is a Research Fellow in the University of Nottingham Biodiscovery Institute, Nottingham, UK
– An excellent very readable summary of the present situation regarding the modulators including the key references
Jane C Davies , Claire E Wainwright , Gregory S Sawicki , Mark N Higgins, Daniel Campbell, Christopher Harris , Paul Panorchan , Eric Haseltine , Simon Tian , Margaret Rosenfeld , ARRIVAL Study Group. Ivacaftor in Infants Aged 4 to <12 Months With Cystic Fibrosis and a Gating Mutation: Results of a 2-Part Phase 3 Clinical Trial. Am J Respir Crit Care Med 2020 Oct 7. doi: 10.1164/rccm.202008-3177OC.Online ahead of print.[Pubmed]

Jane Davies
Rationale: We previously reported that ivacaftor was safe and well tolerated in cohorts aged 12 to <24 months with cystic fibrosis and gating mutations in the ARRIVAL study; here we report results for cohorts aged 4 to <12 months. Objectives: Evaluate safety, pharmacokinetics, and pharmacodynamics of ivacaftor in infants aged 4 to <12 months with ≥1 gating mutation.
Methods: ARRIVAL is a single-arm phase 3 study. Infants received ivacaftor 25 or 50 mg every 12 hours based on age and weight for 4 days in part A and 24 weeks in part B.
Measurements and Main Results: Primary endpoints were safety (parts A and B) and pharmacokinetics (part A). Secondary/tertiary endpoints (part B) included pharmacokinetics and changes in sweat chloride levels, growth, and markers of pancreatic function. Twenty-five infants received ivacaftor, 12 in part A and 17 in part B (4 participated in both). Pharmacokinetics was consistent with that in older groups. Most adverse events were mild/moderate. In part B, cough was the most common adverse event (n=10 [58.8%]). Five infants (part A: n=1 [8.3%]; part B: n=4 [23.5%]) had serious adverse events, all considered not/unlikely related to ivacaftor. No deaths or treatment discontinuations occurred. One infant (5.9%) experienced an alanine transaminase elevation >3 to ≤5 × upper limit of normal at week 24. No other adverse trends in laboratory tests, vital signs, or electrocardiogram parameters were reported. Sweat chloride levels and measures of pancreatic obstruction improved.
Conclusions: This study of ivacaftor in the first year of life supports treating the underlying cause of cystic fibrosis in children aged ≥4 months with ≥1 gating mutation. Clinical trial registration available at www.clinicaltrials.gov, ID:NCT02725567.
Prof Jane Davies is at the National Heart & Lung Institute, Imperial College London and Royal Brompton Hospital, London, United Kingdom of Great Britain and Northern Ireland; j.c.davies@imperial.ac.uk.
Davies JC, Sermet-Gaudelus I, Naehrlich L, Harris RS, Campbell D, Ahluwalia N, Short C, Haseltine E, Panorchan P, Saunders C, Owen CA, Wainwright CE; VX16-661-115 Investigator Group. A phase 3, double-blind, parallel-group study to evaluate the efficacy and safety of tezacaftor in combination with ivacaftor in participants 6 through 11 years of age with cystic fibrosis homozygous for F508del or heterozygous for the F508del-CFTR mutation and a residual function mutation. J Cyst Fibros. 2020 Sep 21:S1569-1993(20)30811-0. doi: 10.1016/j.jcf.2020.07.023. Online ahead of print.Free article. [Pubmed]
Background: The CFTR modulator tezacaftor/ivacaftor was efficacious and generally safe and well tolerated in Phase 3 studies in participants ≥12 years of age with cystic fibrosis (CF) homozygous for the F508del-CFTR mutation or heterozygous with a residual function-CFTR mutation (F/F or F/RF respectively). We evaluated tezacaftor/ivacaftor’s efficacy and safety over 8 weeks in participants 6 through 11 years of age with these mutations.
Methods: Participants were randomized 4:1 to tezacaftor/ivacaftor or a blinding group (placebo for F/F, ivacaftor for F/RF). The primary endpoint was within-group change from baseline in the lung clearance index 2·5 (LCI2·5) through Week 8. Secondary endpoints were change from baseline in sweat chloride (SwCl), cystic fibrosis questionnaire-revised (CFQ-R) respiratory domain score, and safety.
Results: Sixty-seven participants received at least one study drug dose. Of those, 54 received tezacaftor/ivacaftor (F/F, 42; F/RF, 12), 10 placebo, and 3 ivacaftor; 66 completed the study. The within-group change in LCI2·5 was significantly reduced (improved) by -0·51 (95% CI: -0·74, -0·29). SwCl concentration decreased (improved) by -12·3 mmol/L and CFQ-R respiratory domain score increased (improved, nonsignificantly) by 2·3 points. There were no serious adverse events (AEs) or AEs leading to tezacaftor/ivacaftor discontinuation or interruption. The most common AEs (≥10%) in participants receiving tezacaftor/ivacaftor were cough, headache, and productive cough.
Conclusions: Tezacaftor/ivacaftor improved lung function (assessed using LCI) and CFTR function (measured by SwCl concentration) in participants 6 through 11 years of age with F/F or F/RF genotypes. Tezacaftor/ivacaftor was safe and well tolerated; no new safety concerns were identified.
Dr Jane Davies is Professor at the National Heart and Lung Institute, Imperial College London, London, United Kingdom; Royal Brompton & Harefield NHS Foundation Trust, London, United Kingdom
Danahay HL, Lilley S, Fox R, Charlton H, Sabater J, Button B, McCarthy C, Collingwood SP, Gosling M. TMEM16A Potentiation: A Novel Therapeutic Approach for the Treatment of Cystic Fibrosis. Am J Respir Crit Care Med. 2020 Jan 3. doi: 10.1164/rccm.201908-1641OC. [Epub ahead of print] [Pubmed]

Henry Donahay
Rationale: Enhancing non-CFTR mediated anion secretion is an attractive therapeutic approach for the treatment of cystic fibrosis and other muco-obstructive diseases. Objectives: To determine the effects of TMEM16A potentiation upon epithelial fluid secretion and mucociliary clearance. Methods: The effects of a novel low molecular weight TMEM16A potentiator (ETX001) were evaluated in human cell and animal models of airway epithelial function and mucus transport. Measurements & Main Results: Potentiating the activity of TMEM16A with ETX001 increased the Ca2+-activated Cl- channel activity and anion secretion in human bronchial epithelial cells from cystic fibrosis patients without impacting on calcium signalling. ETX001 rapidly increased fluid secretion and airway surface liquid height in cystic fibrosis human bronchial epithelial cells under both static and conditions designed to mimic the shear stress associated with tidal breathing. In ovine models of mucus clearance (tracheal mucus velocity and mucociliary clearance), inhaled ETX001 was able to accelerate clearance both when CFTR function was reduced by administration of a pharmacological blocker and when CFTR was fully functional. Conclusions: Enhancing the activity of TMEM16A increases epithelial fluid secretion and enhances mucus clearance independent of CFTR function. TMEM16A potentiation is a novel approach for the treatment of patients with cystic fibrosis and non-CF muco-obstructive diseases. This article is open access and distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives License 4.0 (http://creativecommons.org/licenses/by-nc-nd/4.0/)
De Jong E, Garratt LW, Looi K, Lee AHY, Ling KM, Smith ML, Falsafi R, Sutanto EN, Hillas J, Iosifidis T, Martinovich KM, Shaw NC, Montgomery ST, Kicic-Starcevich E, Lannigan FJ, Vijayasekaran S, Hancock REW, Stick SM, Kicic A; WAERP, Arest CF. Ivacaftor or lumacaftor/ivacaftor treatment does not alter the core CF airway epithelial gene response to rhinovirus. J Cyst Fibros. 2020 Jul 17:S1569-1993(20)30796-7. doi: 10.1016/j.jcf.2020.07.004. Online ahead of print. [Pubmed]

Emma De Jong
Background: Aberrant responses by the cystic fibrosis airway epithelium during viral infection may underly the clinical observations. Whether CFTR modulators affect antiviral responses by CF epithelia is presently unknown. We tested the hypothesis that treatment of CF epithelial cells with ivacaftor (Iva) or ivacaftor/lumacaftor (Iva/Lum) would improve control of rhinovirus infection.
Methods: Nineteen CF epithelial cultures (10 homozygous for p.Phe508del as CFTR Class 2, 9 p.Phe508del/p.Gly551Asp as Class 3) were infected with rhinovirus 1B at multiplicity of infection 12 for 24 h. Culture RNA and supernatants were harvested to assess gene and protein expression respectively.
Results: RNA-seq analysis comparing rhinovirus infected cultures to control identified 796 and 629 differentially expressed genes for Class 2 and Class 3, respectively. This gene response was highly conserved when cells were treated with CFTR modulators and were predicted to be driven by the same interferon-pathway transcriptional regulators (IFNA, IFNL1, IFNG, IRF7, STAT1). Direct comparisons between treated and untreated infected cultures did not yield any differentially expressed genes for Class 3 and only 68 genes for Class 2. Changes were predominantly related to regulators of lipid metabolism and inflammation, aspects of epithelial biology known to be dysregulated in CF. In addition, CFTR modulators did not affect viral copy number, or levels of pro-inflammatory cytokines produced post-infection.
Conclusions: Though long-term clinical data is not yet available, results presented here suggest that first generation CFTR modulators do not interfere with core airway epithelial responses to rhinovirus infection. Future work should investigate the latest triple modulation therapies
Emma de Jong is at Senior Research Officer at the Telethon Kids Institute Respiratory Research Centre, Nedlands, 6009, Western Australia, Australia.
Hugo R de Jonge , Maria C Ardelean , Marcel Jc Bijvelds , Paola Vergani Strategies for cystic fibrosis transmembrane conductance regulator inhibition: from molecular mechanisms to treatment for secretory diarrhoeas. FEBS Lett . 2020 Oct 28. doi: 10.1002/1873-3468.13971. Online ahead of print. [Pubmed]

Hugo de Jonge
Cystic fibrosis transmembrane conductance regulator (CFTR) is an unusual ABC transporter. It acts as an anion-selective channel that drives osmotic fluid transport across many epithelia. In the gut, CFTR is crucial for maintaining fluid and acid-base homeostasis, and its activity is tightly controlled by multiple neuro-endocrine factors. However, microbial toxins can disrupt this intricate control mechanism and trigger protracted activation of CFTR. This results in the massive faecal water loss, metabolic acidosis and dehydration that characterise secretory diarrhoeas, a major cause of malnutrition and death of children under 5 years of age. Compounds that inhibit CFTR could improve emergency treatment of diarrhoeal disease. Drawing on recent structural and functional insight, we discuss how existing CFTR inhibitors function at the molecular and cellular level. We compare their mechanisms of action to those of inhibitors of related ABC transporters, revealing some unexpected features of drug action on CFTR. Although challenges remain, especially relating to the practical effectiveness of currently available CFTR inhibitors, we discuss how recent technological advances might help develop therapies to better address this important global health need.
Dr Hugo R de Jonge is a scientist in the Department of Gastroenterology & Hepatology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Dr Henry Danahay is co-founder of Enterprise Therapeutics Ltd, Brighton, head of biology and Hon. Senior Research Fellow University of Sussex
DiMango E, Overdevest J, Keating C, Francis SF, Dansky D, Gudis D. Effect of highly effective modulator treatment on sinonasal symptoms in cysticfibrosis. J Cyst Fibros. 2020 Jul 18:S1569-1993(20)30794-3. doi: 10.1016/j.jcf.2020.07.002. Online ahead of print. [Pubmed]

Emily DiMango
Elexacaftor-tezacaftor-ivacaftor is a highly effective modulator for cystic fibrosis (CF) patients homozygous or heterozygous for F508del. Effects of the drug on sinonasal symptoms have not been studied Adult participants were prospectively evaluated at baseline and after three months of treatment using validated questionnaires assessing sinonasal symptoms (SNOT-22) and CF-related quality of life (CFQ-R).
Results: Forty-three participants completed the study; 23 were taking other CF transmembrane conductance (CFTR) modulators at the time of study participation. There was a significant improvement in mean SNOT-22 from 34.8 (29.4-40, 95% confidence interval) to 24.4 (19.9-29.0) (p = 0.000003) and in the Respiratory domain of the CFQR from 60.6 (57.1-64.1) to 83.3 (79.4-87.2) (p = 0.0000002), both achieving a minimal clinically important difference. Patients previously taking CFTR modulators experienced a greater benefit in sinonasal and respiratory symptoms.
Conclusions: Elexacaftor-tezacaftor-ivacaftor is associated with significant improvement in sinonasal symptoms; previous use of CFTR modulators is associated with greater benefit
Dr Emily DiMango is Professor of Medicine at Columbia University Medical Center; Director, John Edsall-John Wood Asthma Center; Director, Adult Cystic Fibrosis Program
de Vries JM, Green D, Kucera JN, Fabbrini AL, Kidder M, Brown J, Wilsey M. Cystic Fibrosis-Related Pancreatic Cysts Decrease in Size and Number Upon Treatment With Cystic Fibrosis Transmembrane Conductance Regulator Modulators. Pancreas. 2020;49(6):e50-e51. doi:10.1097/MPA.0000000000001567 [Pubmed]

Michael Wilsey
No access to abstract at time of reviewing – although the title contains the message!
Dr J M de Vries is in the Department of Pediatric Gastroenterology and Nutrition, Johns Hopkins All Children’s Hospital.
Dr. Wilsey specializes in pediatric gastroenterology, hepatology and nutrition in the Department of Medicine. He became chief of the medical staff in January 2020 and is vice chair of the Department of Gastroenterology
Egan ME. Emerging Technologies for Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Restoration in all People with CF. Pediatr Pulmonol. 2020 Jul 18. doi: 10.1002/ppul.24965. Online ahead of print.[Pubmed]

Mary Egan
These therapies optimize the function of the patients’ endogenous mutant Cystic FibrosisTransmembrane Conductance Regulator (CFTR), which results in return of CFTR channel function. 2019 will be remembered as a landmark year for the Cystic Fibrosis (CF) community because it marks the year when effective modulator therapy became available for most CF patients. It is unlikely that this approach will provide a game changing therapy for all. In part because the response to even the most promising modulator therapy is variable and an area of active investigation. Also, there are about 10% of patients with CF who don’t produce a mutant protein to modulate, potentiate or optimize and for these patients such therapies are unlikely to be of significant benefit. Efforts to develop small molecule therapy to promote protein production in patients with nonsense mutations such as PTC124 has proved to be far more challenging than predicted.
There is a need to develop new therapeutic approaches that can work for this patient population. These new therapies will be genetic-based therapies that include ribonucleic acid (RNA) therapies, deoxyribonucleic acid (DNA) therapies and gene editing technologies. Each approach will result in functional CFTR expression in CF affected cells. Ideally, these approaches would require less frequent dosing than effective modulators, which are given daily. For instance, RNA based treatments could be given periodically.
Ultimately, treatment with certain gene-altering treatments could be given once in a lifetime and lead to a permanent cure. In this review which is based on Plenary 1 from the North American Cystic Fibrosis Conference in 2019 which is based on Plenary 1 from the North American Cystic Fibrosis Conference in 2019 the potential of RNA therapies, gene transfer therapies and gene editing therapies for the treatment of CF are reviewed, as well as the challenges that will need to be faced as we harness the power of these emerging therapies towards a one-time cure.
Dr Marie E Egan is Professor of Pediatrics (Respiratory) and of Cellular and Molecular Physiology; Director Cystic fibrosis Center and Vice Chair of Research Pediatrics, School of Medicine, Yale University, New Haven, CT, USA.
Ryosuke Fukuda, Tsukasa Okiyoneda. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitylation as a Novel Pharmaceutical Target for Cystic Fibrosis. Pharmaceuticals (Basel) 2020 Apr 22;13(4):E75. doi: 10.3390/ph13040075 Full text [Pubmed]

Tsukasa Okiyoneda

Ryosuke Fukuda
Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene decrease the structural stability and function of the CFTR protein, resulting in cystic fibrosis. Recently, the effect of CFTR-targeting combination therapy has dramatically increased, and it is expected that add-on drugs that modulate the CFTR surrounding environment will further enhance their effectiveness. Various interacting proteins have been implicated in the structural stability of CFTR and, among them, molecules involved in CFTR ubiquitylation are promising therapeutic targets as regulators of CFTR degradation. This review focuses on the ubiquitylation mechanism that contributes to the stability of mutant CFTR at the endoplasmic reticulum (ER) and post-ER compartments and discusses the possibility as a pharmacological target for cystic fibrosis (CF)
Ryosuke Fukuda is Research Associate in the Department of Biomedical Chemistry, School of Science and Technology, Kwansei Gakuin University, Japan.
Tsukasa Okiyoneda is Associate Professor, Kwansei Gakuin University
(Ubiquitination: The “kiss of death” process for a protein. In ubiquitination, a protein is inactivated by attaching ubiquitin to it. Ubiquitin is a small molecule. It acts as a tag that signals the protein-transport machinery to ferry the protein to the proteasome for degradation).
Monica Gelzo, Paola Iacotucci, Mafalda Caputo, Gustavo Cernera, Marika Comegna, Vincenzo Carnovale, Gaetano Corso, Giuseppe Castaldo. Lumacaftor/ivacaftor improves liver cholesterol metabolism but does not influence hypocholesterolemia in patients with cystic fibrosis. J Cyst Fibros 2020 Jun 22;S1569-1993(20)30779-7.doi: 10.1016/j.jcf.2020.06.015.Online ahead of print. [Pubmed]
Cystic fibrosis (CF) patients have reduced intestinal absorption of sterols and, despite enhanced endogenous synthesis, low plasma cholesterol. Lumacaftor/ivacaftor (ORKAMBI) CFTR protein modulator therapy is used to improve the clinical outcome of CF patients homozygous for F508del mutation (homo-deltaF508).Aim of this study is to evaluate the cholesterol metabolism and hepatobiliary injury/function in adult homo-deltaF508 patients, before and after lumacaftor/ivacaftor treatment. Baseline parameters in homo-deltaF508 patients were compared to those in CF patients compound heterozygous for F508del mutation and another severe mutation (hetero-deltaF508).
Methods: Cholesterol metabolism was evaluated measuring plasma phytosterols and cholestanol, as intestinal absorption markers, and lathosterol, as liver biosynthesis marker. We quantified serum vitamin E, as nutritional marker. We evaluated liver injury by aspartate aminotransferase (AST) and alanine transaminase (ALT), biliary injury by γ-glutamyltransferase (γGT) and AP, and the liver function by bilirubin and albumin.
Results: Before the treatment, homo-deltaF508 patients (n = 20) had significantly lower cholesterol and vitamin E compared to hetero-deltaF508 (n = 20). Lumacaftor/ivacaftor (ORKAMBI) treatment caused: 1) further reduction of cholesterol; 2) lathosterol reduction, suggesting a normalization of endogenous synthesis; 3) cholestanol and vitamin E increment, indicating an improvement of lipid digestion/absorption. Vitamin E difference (after-before treatment) was positively associated to treatment months. Alkaline phosphatase was also reduced.
Conclusions: These data suggest an effect of lumacaftor/ivacaftor on cholesterol metabolism and enterohepatic flux in CF patients. However, lumacaftor/ivacaftor (ORKAMBI) does not promote the increase of cholesterol serum concentration that on the contrary declines. Further studies are needed to research the real mechanism causing this reduction.
Monica Geizo is in the Dipartimento di Medicina Molecolare e Biotecnologie Mediche, Università di Napoli Federico II, Naples, Italy; CEINGE – Biotecnologie avanzate, Naples, Italy.
George A, Smith B, Sawicki GS, Goetz DM. Survey of Patients with Cystic Fibrosis and Caregivers Decisions Regarding CFTR Modulators [published online ahead of print, 2020 Jun 26]. Pediatr Pulmonol. 2020;10.1002/ppul.24926. doi:10.1002/ppul.24926 [Pubmed]
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulators are a novel approach to CF management that has become more readily available chronic CF therapies for certain populations of patients with CF. A cross-sectional survey of adults with CF and caregivers of pediatric patients with CF was done in two CF Centers to better understand the decision-making process including the potential influence of social media, CF care-teams, and family members on their decision whether to begin a CFTR modulator.
For the 90 participants, the most common influences in the decision to start modulator therapy were the CF providers/care teams (n=63), parents (n=49) and individuals with CF (n=27). The most impactful influence in the decision-making process were providers/care team (n=47) and parents (n=18). Social media was an influence for only 12 respondents, with an overall positive impact. Information from the CF Foundation was an influence for 12 participants and the main influence for 6 participants.
The most common reasons for stopping LUM-IVA were having TEZ-IVA as an option (n=25) and side-effects (n=15). Family and CF clinicians were the two main influences on the decision to initiate modulator therapy. CF clinicians were seen to be the most influential source. Social media had less influence on the decision-making process than expected despite the wide presence of the CF community online.
Dr Ashish George is in the Dept of Pediatrics, University at Buffalo, Buffalo, NY, United States.
Elizabeth Marie Gavioli, Nerli Guardado, Farah Haniff, Nouran Deiab, Etty Vider. A current review of the safety of cystic fibrosis transmembrane conductance regulator modulators. J Clin Pharm Ther 2020 Dec 7.doi: 10.1111/jcpt.13329. Online ahead of print. [Pubmed]
What is known and objective: Treatment with cystic fibrosis transmembrane conductance regulator (CFTR) modulators has led to improved clinical outcomes and an increase in lifespans of cystic fibrosis (CF) patients. As CF patients continue to live longer, they are at risk for developing adverse drug reactions associated with polypharmacy and CFTR modulators.
Comment: The authors aim to describe safety concerns of the current combination CFTR modulators, based upon a literature review, including notable safety concerns and recommendations for drug-drug interactions.
What is new and conclusion: Cystic fibrosis transmembrane conductance regulator agents are generally well tolerated with low discontinuation rates when compared to placebo. Elevations in liver enzymes and drug-drug interactions are the most notable safety concerns. Additionally, lumacaftor/ivacaftor has shown more respiratory-related adverse events and drug-drug interactions compared to elexacaftor/tezacaftor/ivacaftor and tezacaftor/ivacaftor. Postmarketing studies are needed to determine long-term safety concerns.
Elizabeth Marie Gavioli is assistant professor of pharmacy practice at the Arnold and Marie Schwartz College of Pharmacy and Health Sciences, Brooklyn, NY, USA.
George A, Smith B, Sawicki GS, Goetz DM. Survey of Patients with Cystic Fibrosis and Caregivers Decisions Regarding CFTR Modulators [published online ahead of print, 2020 Jun 26]. Pediatr Pulmonol. 2020;10.1002/ppul.24926. doi:10.1002/ppul.24926 [Pubmed]
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulators are a novel approach to CF management that has become more readily available chronic CF therapies for certain populations of patients with CF. A cross-sectional survey of adults with CF and caregivers of pediatric patients with CF was done in two CF Centers to better understand the decision-making process including the potential influence of social media, CF care-teams, and family members on their decision whether to begin a CFTR modulator.
For the 90 participants, the most common influences in the decision to start modulator therapy were the CF providers/care teams (n=63), parents (n=49) and individuals with CF (n=27). The most impactful influence in the decision-making process were providers/care team (n=47) and parents (n=18). Social media was an influence for only 12 respondents, with an overall positive impact. Information from the CF Foundation was an influence for 12 participants and the main influence for 6 participants.
The most common reasons for stopping LUM-IVA were having TEZ-IVA as an option (n=25) and side-effects (n=15). Family and CF clinicians were the two main influences on the decision to initiate modulator therapy. CF clinicians were seen to be the most influential source. Social media had less influence on the decision-making process than expected despite the wide presence of the CF community online.
Dr Ashish George is in the Dept of Pediatrics, University at Buffalo, Buffalo, NY, United States.
Geurts MH, de Poel E, Amatngalim GD, Oka R, Meijers FM, Kruisselbrink E, van Mourik P, Berkers G, de Winter-de Groot KM, Michel S, Muilwijk D, Aalbers BL, Mullenders J, Boj SF, Suen SWF, Brunsveld JE, Janssens HM, Mall MA, Graeber SY, van Boxtel R, van der Ent CK, Beekman JM, Clevers H. CRISPR-Based Adenine Editors Correct Nonsense Mutations in a Cystic Fibrosis Organoid Biobank.
Cell Stem Cell. 2020 Feb 13. pii: S1934-5909(20)30019-9. doi: 10.1016/j.stem.2020.01.019. [Epub ahead of print][Pubmed]

Maarten Geurts

Hans Clevers
Adenine base editing (ABE) enables enzymatic conversion from A-T into G-C base pairs. ABE holds promise for clinical application, as it does not depend on the introduction of double-strand breaks, contrary to conventional CRISPR/Cas9-mediated genome engineering. Here, the authors describe a cystic fibrosis (CF) intestinal organoid biobank, representing 664 patients, of which ~20% can theoretically be repaired by ABE. We apply SpCas9-ABE (PAM recognition sequence: NGG) and xCas9-ABE (PAM recognition sequence: NGN) on four selected CF organoid samples. Genetic and functional repair was obtained in all four cases, while whole-genome sequencing (WGS) of corrected lines of two patients did not detect off-target mutations. These observations exemplify the value of large, patient-derived organoid biobanks representing hereditary disease and indicate that ABE may be safely applied in human cells.
Dr Maarten Geurts is a PhD student at the Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences (KNAW) and University Medical Center Utrecht, the Netherlands and working in the Clevers Group
Professor Hans Clevers is leader of the Clevers Group and Chief Scientific Officer/Director Research of the Princess Máxima Center for pediatric oncology, Utrecht
Guimbellot JS, Ryan KJ, Anderson JD, Liu Z, Kersh L, Esther CR, Rowe SM, Acosta EP. Variable cellular ivacaftor concentrations in people with cystic fibrosis on modulator therapy. J Cyst Fibros. 2020 Feb 7. pii: S1569-1993(20)30035-7. doi: 10.1016/j.jcf.2020.01.011. [Epub ahead of print) [Pubmed]

Edward P Acosta

Jennifer S Guimbellot
The development of CFTR modulators has transformed the care of patients with cystic fibrosis(CF). Although the clinical efficacy of modulators depends on their concentrations in target tissues, the pharmacokinetic properties of these drugs in epithelia are not utilized to guide patient care. The authors developed assays to quantitate ivacaftor in cells and plasma from patients on modulator therapy, and analyses revealed that cellular ivacaftor concentrations differ from plasma concentrations measured concurrently, with evidence of in vivo accumulation of ivacaftor in the cells of patients.
While the nature of this study is exploratory and limited by a small number of patients, these findings suggest that techniques to measure modulator concentrations in vivo will be essential to interpreting their clinical impact, particularly given the evidence that ivacaftor concentrations influence the activity and stability of restored CFTR protein.
Dr Jennifer S Guimbellot is a respiratory paediatrician at the Gregory Fleming James Cystic Fibrosis Research Center, University of Alabama and the Department of Pediatrics, Division of Pulmonary and Sleep Medicine, UAB, Birmingham, Alabama.,
Professor Edward Acosta is Professor; Director, Division of Clinical Pharmacology and the Antiviral Pharmacology laboratory in the University of Alabama, Birmingham
Gostelie R, Stegeman I, Berkers G, Bittermann J, Ligtenberg-van der Drift I, Kipshagen PV, de Winter-de Groot K, Speleman L. The impact of ivacaftor on sinonasal pathology in S1251N-mediated cysticfibrosis patients. PLoS One. 2020 Jul 20;15(7):e0235638. doi: 10.1371/journal.pone.0235638. eCollection 2020 Free article. [Pubmed]
Eight patients with cystic fibrosis with an S1251N mutation, treated with the potentiator ivacaftor were investigated. Ivacaftor (Kalydeco, VX-770) therapy. Computed tomography imaging of paranasal sinuses. Nasal nitric oxide concentration measurements and nasal endoscopy. Primary outcome is opacification of paranasal sinuses examined with computed tomography scan analysis and scaled by the modified Lund-Mackay score before and one year after treatment. Secondary outcomes are nasal nitric oxide concentration levels, sinonasal symptoms and nasal endoscopic findings before and approximately two months and in some cases one year after treatment.
Results: Computed tomography scan analysis showed a significant decrease in opacification of the majority of paranasal sinuses comparing the opacification score per paranasal sinus before and after one year of treatment with ivacaftor. Median nasal nitric oxide levels significantly improved from 220.00 (IQR:136.00-341.18) to 462.84 (IQR:233.17-636.25) (p = 0.017) parts per billion. Likewise, the majority of sinonasal symptoms and nasal endoscopic pathology decreased or resolved at two months after the use of ivacaftor.
Conclusion and relevance: Ivacaftor appears to improve sinonasal outcome parameters and thereby sinonasal health in patients with cystic fibrosis with an S1251N mutation.
Fromm the University Medical Center, Utrecht University, Utrecht, The Netherlands.
Guhr Lee TN, Cholon DM, Quinney NL, Gentzsch M, Esther CR Jr. Accumulation and persistence of ivacaftor in airway epithelia with prolonged treatment. J Cyst Fibros. 2020 Jun 11:S1569-1993(20)30123-5. doi: 10.1016/j.jcf.2020.04.010. Online ahead of print.[Pubmed]

Tara Guhr Lee
Current dosing strategies of CFTR modulators are based on serum pharmacokinetics, but drug concentrations in target tissues such as airway epithelia are not known. Previous data suggest that CFTR modulators may accumulate in airway epithelia, and serum pharmacokinetics may not accurately predict effects of chronic treatment.
CF (F508del homozygous) primary human bronchial epithelial (HBE) cells grown at air-liquid interface were treated for 14 days with ivacaftor plus lumacaftor or ivacaftor plus tezacaftor, followed by a 14-day washout period. At various intervals during treatment and washout phases, drug concentrations were measured via mass spectrometry, electrophysiological function was assessed in Ussing chambers, and mature CFTR protein was quantified by Western blotting.
During treatment, ivacaftor accumulated in CF-HBEs to a much greater extent than either lumacaftor or tezacaftor and remained persistently elevated even after 14 days of washout. CFTR activity peaked at 7 days of treatment but diminished with further ivacaftor accumulation, though remained above baseline even after washout.
Conclusions: Intracellular accrual and persistence of CFTR modulators during and after chronic treatment suggest complex pharmacokinetic and pharmacodynamic properties within airway epithelia that are not predicted by serum pharmacokinetics. Direct measurement of drugs in target tissues may be needed to optimize dosing strategies, and the persistence of CFTR modulators after treatment cessation has implications for personalized medicine approaches.
Dr Tara Nicole Guhr Lee is a Research Technician in the Division of Pediatric Pulmonology, Department of Pediatrics, University of North Carolina School of Medicine.
Jonathan L Gillan , Donald J Davidson, Robert D Gray. Targeting Cystic Fibrosis Inflammation in the Age of CFTR Modulators: Focus on Macrophages. Eur Respir J 2020 Dec 10;2003502.doi: 10.1183/13993003.03502-2020.Online ahead of print.[Pubmed]

Robert B Gray
Cystic fibrosis is a life-shortening, multiorgan, autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The most prominent clinical manifestation in CF is the development of progressive lung disease characterised by an intense, chronic inflammatory airway response that culminates in respiratory failure, and ultimately death. In recent years, a new class of therapeutics which have the potential to correct the underlying defect in CF, known as CFTR modulators, have revolutionised the field. Despite the exciting success of these drugs, their impact on airway inflammation, and its long-term consequences, remain undetermined. In addition, studies querying the absolute requirement for infection as a driver of CF inflammation have challenged the traditional consensus on CF pathogenesis, and also emphasise the need to prioritise complementary anti-inflammatory treatments in CF. Macrophages, often overlooked in CF research despite their integral role in other chronic inflammatory pathologies, have increasingly become recognised as key players in the initiation, perpetuation, and resolution of CF lung inflammation, perhaps as a direct result of CFTR dysfunction. These findings suggest that macrophages may be an important target for novel anti-inflammatory interventional strategies to effectively treat CF lung function decline. This review will consider evidence for the efficacy for anti-inflammatories in the treatment of CF, the potential role of macrophages, and the significance of targeting these pathways at a time when rectifying the basic defect in CF, through use of novel CFTR modulator therapies, is becoming increasingly viable.
Jonathan L Gillian is a PhD student and Dr Robert Gray is NRS Senior Clinical Fellow & Hon. Consultant Respiratory Physician at the University of Edinburgh Centre for Inflammation Research, Queen’s Medical Research Institute, Edinburgh, UK.
Hahn A, Burrell A, Ansusinha E, Peng D, Chaney H, Sami I, Perez GF, Koumbourlis AC, McCarter R, Freishtat RJ, Crandall KA, Zemanick ET. Airway microbial diversity is decreased in young children with cystic fibrosiscompared to healthy controls but improved with CFTR modulation. Heliyon. 2020 Jun 1;6(6):e04104. doi: 10.1016/j.heliyon.2020.e04104. eCollection 2020 Jun. Free PMC article. [Pubmed]

Andrea Hahn
The study objective was to test for differences in the upper airway microbiome of children with CF and healthy controls and age-related differences in children with CF.
Oropharyngeal swabs and clinical data were obtained from 25 children with CF and 50 healthy controls aged ≤6 years. Bacterial DNA was amplified and sequenced for the V4 region of 16S rRNA marker-gene. Alpha diversity was measured using operational taxonomic units (OTUs), Shannon diversity, and the inverse Simpson’s index. Beta diversity was measured using Morisita-Horn and Bray-Curtis and Jaccard distances. General linear models were used for comparison of alpha diversity measures between groups to account for differences in demographics and exposures. Mixed effects general linear models were used for longitudinal comparisons 1) between children with CF of different ages and 2) between children with CF receiving CF transmembrane conductance regulator (CFTR) modulators, children with CF not receiving CFTR modulators, and healthy controls to adjust for repeated measures per subject.
Children with CF were more likely to have received antibiotics in the prior year than healthy controls (92% vs 24%, p < 0.001). Controlling age, race, ethnicity, length of breastfeeding, and having siblings, children with CF had a lower richness than healthy controls: OTUs 62.1 vs 83, p = 0.022; and trended toward lower diversity: Shannon 2.09 vs 2.35, p = 0.057; inverse Simpson 5.7 vs 6.92, p = 0.118. Staphylococcus, three Rothia OTUs, and two Streptococcus OTUs were more abundant in CF children versus healthy controls (all p < 0.05). Bray-Curtis and Jaccard distances, which reflect overall microbial community composition, were also significantly different (both p = 0.001). In longitudinally collected samples from children with CF, Morisita-Horn trended toward more similarity in those aged 0-2 years compared to those aged 3-6 years (p = 0.070). In children >2 years of age, there was a significant trend in increasing alpha diversity measures between children with CF not receiving CFTR modulators, children with CF receiving CFTR modulators, and healthy controls: OTUs 63.7 vs 74.7 vs 97.6, p < 0.001; Shannon 2.11 vs 2.34 vs 2.56, p < 0.001; inverse Simpson 5.78 vs 7.23 vs 7.96, p < 0.001.
Conclusions: Children with CF have lower bacterial diversity and different composition of organisms compared with healthy controls. This appears to start in early childhood, is possibly related to the use of antibiotics, and may be partially corrected with the use of CFTR modulators.
Dr Andrea Hahn is an infectious disease specialist and investigator in the Division of Infectious Diseases, Children’s National Hospital, Washington, DC, USA.
Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, Rowe SM, Sagel SD. Changes in Airway Microbiome and Inflammation with Ivacaftor Treatment in Patients with Cystic Fibrosis and the G551D Mutation. Ann Am Thorac Soc. 2020 Feb;17(2):212-220. doi: 10.1513/AnnalsATS.201907-493OC.[Pubmed]
It remains unclear how improving CFTR function modifies existing airway infection and inflammation. This study aimed to compare sputum microbiome and markers of inflammation before and after 6 months of ivacaftor treatment. The study included 31 people with CF, ages 10 years and older, with at least one G551D CFTR allele and an forced expiratory volume in 1 second (FEV1) of 40% predicted or greater who were enrolled in the GOAL (G551D Observational) study. Sputum samples were collected either by induction (n = 14) or by spontaneous expectoration (n = 17) before and 6 months after initiation of ivacaftor. Changes in bacterial community indices by sequencing of 16S rRNA amplicons, total and specific bacterial load, and a panel of proteases, antiproteases, and inflammatory cytokines were determined.
The cohort that spontaneously expectorated sputum had a lower FEV1, a higher proportion with Pseudomonas aeruginosa infection, and higher concentrations of sputum inflammatory markers compared with the cohort that provided sputum by induction, no significant changes in bacterial diversity, specific bacterial pathogens, or markers of inflammation were observed in these subjects. Neither total bacterial load nor presence of Pseudomonas changed significantly between paired samples with ivacaftor treatment. Younger patients experienced more shifts in their microbial communities than older patients.
The authors concluded 6 months of ivacaftor treatment were not associated with significant changes in airway microbial communities or measures of inflammation. These data suggest that concomitant antimicrobial and anti-inflammatory treatments will still be needed to manage airway disease in patients with CF treated with highly effective CFTR modulator therapy, especially in older patients with more advanced disease.
-These findings are not unexpected as the situation in the airways will have passed from the uninfected CFTR state to the chronic progressive inflammatory phase where restoration of CFTR will not affected the progression of the damaging chronic inflammation.
Katherine B Hisert, Timothy P Birkland Kelly Q Schoenfelt, Matthew E Long, Brenda Grogan, Suzanne Carter, W Conrad Liles, Edward F McKone, Lev Becker, Anne M Manicone Ivacaftor Decreases Monocyte Sensitivity to Interferon-γ in People with Cystic Fibrosis. ERJ Open Res 2020 Apr 19;6(2):00318-2019.doi: 10.1183/23120541.00318-2019.eCollection 2020 Apr.[Pubmed] (http://bit.ly/2TeI6LG) Free article
This study demonstrates that initiation of the CFTR modulator ivacaftor in people with cystic fibrosis and susceptible CFTR mutations causes an acute reduction in blood monocyte sensitivity to the key proinflammatory cytokine IFN-γ ……..”Studies examining individuals before and after initiation of CFTR modulators have revealed novel functions of CFTR and shown that CFTR modulators do not reverse all disease manifestations [3–5]. Thus, knowledge of the post-modulator cystic fibrosis disease state is crucial for understanding what continued therapies will be needed for people with cystic fibrosis and what new challenges may arise”…….
Dr Katherine Hisert is in the Dept of Medicine, University of Washington, Seattle, WA,
Jarosz-Griffiths HH, Scambler T, Wong CH Lara-Reyna S, Holbrook J, Martinon F, Savic S, Whitaker P, Etherington C, Spoletini G, Clifton I6, Mehta A, McDermott MF, Peckham D. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife. 2020 Mar 2;9. pii: e54556. doi: 10.7554/eLife.54556.[Pubmed] Free full text

Thomas E Scambler

Heledd Jarosz-Griffiths
Previously, we showed that serum and monocytes from patients with CF exhibit an enhanced NLRP3-inflammasome signature with increased IL-18, IL-1β, caspase-1 activity and ASC speck release (Scambler et al. eLife 2019). Here we show that CFTR modulators down regulate this exaggerated proinflammatory response following LPS/ATP stimulation. In vitro application of ivacaftor/lumacaftor or ivacaftor/tezacaftor to CF monocytes showed a significant reduction in IL-18, whereas IL-1β was only reduced with ivacaftor/tezacaftor. Thirteen adults starting ivacaftor/lumacaftor and eight starting ivacaftor/tezacaftor were assessed over three months. Serum IL-18 and TNF decreased significantly with treatments, but IL-1β declined following ivacaftor/tezacaftor. In (LPS/ATP-stimulated) PBMCs, IL-18/TNF/caspase-1 were all significantly decreased and IL-10 was increased with both combinations. Ivacaftor/tezacaftor alone showed a significant reduction in IL-1β and pro-IL-1β mRNA.
This study demonstrates that these CFTR modulator combinations have potent anti-inflammatory properties, in addition to their ability to stimulate CFTR function, which could contribute to improved clinical outcomes.
Co-authors – Dr Heledd H Jarosz-Griffiths is in Leeds Institute of Medical Research at St James’s and the Leeds Cystic Fibrosis Trust Strategic Research Centre, University of Leeds, United Kingdom.
Dr Thomas Scambler of the Institute of Medical Research at St James’s, University of Leeds and the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, United Kingdom.
Comments from the journal Editors, (Jos WM van der Meer and Siroon Bekkering, Radboud University, Netherlands) – “the paper convincingly shows the auto-inflammatory characteristics of CF, and its dependence on NLRP3. The underlying mechanisms have been thoroughly studied. With that, the paper is innovative and provides insights that will be clinically relevant, especially as it will probably have consequences for new treatment modalities”.
Lead authors (Dr Thomas Scambler and Dr Heledd Jarosz-Griffiths, St James’ University Hospital) comment “Blocking the sodium channel (ENaC) in combination with therapies targeting chemical cytokines (IL-18 and IL-1b) which are over-produced in CF is a potential strategy to reduce inflammation in CF, and may help combat the onset of secondary diseases such as diabetes and joint disease.”
Expert Opinion: Globally, nonsense mutations account for ~11% of all described gene lesions causing inherited monogenetic diseases. In CF and nephropathic cystinosis, they comprise from 10-12% of the disease-causative alleles. ELX-02 is in development as a therapeutic for patients with these alleles as in vitro and in vivo data demonstrated dose-dependent read-through of nonsense mutations to produce full-length, functional proteins. Since read-through efficiency varies between alleles, and mRNA context, careful consideration of target patient populations is required. The results to date support the on-going Phase 2 clinical evaluations of ELX-02 as a read-through agent.
Eitan Kerem , Malena Cohen-Cymberknoh, Reuven Tsabari , Michael Wilschanski, Joel Reiter, David Shoseyov, Alex Gileles-Hillel, Thea Pugatsch , Jane C Davies , Christopher Short , ClareSaunders , CynthiaDeSouza James C Sullivan , Jamie R Doyle, Keval Chandarana, . Ivacaftor in People With Cystic Fibrosis and a 3849+10kb C →T or D1152H Residual Function Mutation Ann Am Thorac Soc 2020 Oct 23. doi: 10.1513/AnnalsATS.202006-659OC.Online ahead of print. [Pubmed]
Ivacaftor’s clinical effects in the residual function mutations 3849+10kb C →T and D1152H warrant further characterization.
Objectives: Evaluate ivacaftor’s effect in people with cystic fibrosis aged ≥6 years with 3849+10kb C→T or D1152H residual function mutations; explore the correlation between ivacaftor-induced organoid-based cystic fibrosis transmembrane conductance regulator function measurements and clinical response to ivacaftor.
Methods: Participants were randomized (1:1) in this placebo-controlled crossover study; each treatment sequence included two 8-week treatments with an 8-week washout period. The primary endpoint was absolute change in lung clearance index2.5 from baseline through Week 8. Additional endpoints included lung function, patient-reported outcomes, and in vitro intestinal organoid-based measurements of ivacaftor-induced cystic fibrosis transmembrane conductance regulator function.
Results: Of 38 participants, 37 completed the study. The primary endpoint was met; the Bayesian posterior probability of improvement in lung clearance index2.5 with ivacaftor vs placebo was >99%. Additional endpoints improved with ivacaftor. Safety findings were consistent with ivacaftor’s known safety profile. Dose-dependent swelling was observed in 23/25 viable organoid cultures with ivacaftor treatment. Correlations between ivacaftor-induced organoid swelling and clinical endpoints were negligible to low.
Conclusions: In people with cystic fibrosis aged ≥6 years with a 3849+10kb C →T or D1152H mutation, ivacaftor treatment improved clinical endpoints vs placebo; however, there was no correlation between organoid swelling and change in clinical endpoints. The organoid assay may assist in identification of ivacaftor-responsive mutations but in this study did not predict magnitude of clinical benefit for individual people with cystic fibrosis with these two mutations.
– This failure of the organoid response to predict the magnitude of the response to the ivacaftor is disappointing.
Prof. Eitan Kerem is in the Department of Pediatrics and CF Center, Hadassah Hebrew University Medical Center , Jerusalem, Israel.
Kopp BT, Fitch J, Jaramillo L, Shrestha CL, Robledo-Avila F, Zhang S, Palacios S, Woodley F, Hayes D Jr, Partida-Sanchez S, Ramilo O, White P, Mejias A. Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J Cyst Fibros. 2020 Mar;19(2):245-254. doi: 10.1016/j.jcf.2019.08.021. Epub 2019 Aug 29.[Pubmed]

Benjamin Kopp
Background: Cystic fibrosis (CF) remains without a definitive cure. Novel therapeutics targeting the causative defect in the cystic fibrosis transmembrane conductance regulator (CFTR) gene are in clinical use. Lumacaftor/ivacaftor is a CFTR modulator approved for patients homozygous for the CFTR variant p.Phe508del, but there are wide variations in treatment responses preventing prediction of patient responses. We aimed to determine changes in gene expression related to treatment initiation and response.
Methods: Whole-blood transcriptomics was performed using RNA-Seq in 20 patients with CF pre- and 6 months post-lumacaftor/ivacaftor (drug) initiation and 20 non-CF healthy controls. Correlation of gene expression with clinical variables was performed by stratification via clinical responses.
Results: We identified 491 genes that were differentially expressed in CF patients (pre-drug) compared with non-CF controls and 36 genes when comparing pre-drug to post-drug profiles. Both pre- and post-drug CF profiles were associated with marked overexpression of inflammation-related genes and apoptosis genes, and significant under-expression of T cell and NK cell-related genes compared to non-CF. CF patients post-drug demonstrated normalized protein synthesis expression, and decreased expression of cell-death genes compared to pre-drug profiles, irrespective of clinical response. However, CF clinical responders demonstrated changes in eIF2 signaling, oxidative phosphorylation, IL-17 signaling, and mitochondrial function compared to non-responders. Top overexpressed genes (MMP9 and SOCS3) that decreased post-drug were validated by qRT-PCR. Functional assays demonstrated that CF monocytes normalized calcium (increases MMP9 expression) concentrations post-drug.
Conclusions: Transcriptomics revealed differentially regulated pathways in CF patients at baseline compared to non-CF, and in clinical responders to lumacaftor/ivacaftor.
Dr Benjamin T Kopp is in the Division of Pulmonary Medicine, Nationwide Children’s Hospital, Columbus,
OH, USA; Center for Microbial Pathogenesis, Nationwide Children’s Hospital, Columbus, OH, USA.
Kessler L. Can lumacaftor-ivacaftor reverse glucose-tolerance abnormalities in cystic fibrosis? J Cyst Fibros. 2020 May 5. pii: S1569-1993(20)30126-0. doi: 10.1016/j.jcf.2020.04.013. [Epub ahead of print] [Pubmed]

Laurence Kessler
We are thankful to Manfred Ballmann and his colleagues for their interest in our recently published article [1]. By analyzing the effect of one year of lumacaftor–ivacaftor treatment on metabolic status in 40 cystic fibrosis patients affected by early glucose tolerance abnormalities, we observed an improvement in glucose tolerance, together with favourable changes in weight and pulmonary function. Furthermore, both one- and two-hour glycemia decreased in the subgroup, with improved oral glucose tolerance test (OGTT) categories. In their correspondence, Manfred Ballmann et al. propose that the effects of lumacaftor–ivacaftor should be interpreted with caution, due to the selected study population and natural history of glucose-tolerance abnormalities in cystic fibrosis.
The study population was composed of patients with either initial impaired glucose tolerance (IGT) or newly diagnosed cystic fibrosis-related diabetes (CFRD), since the moderate functional alteration of beta cells is still potentially reversible at this stage of the disease. We agree with Manfred Ballmann because, in our study, we did not consider the potential worsening of glucose tolerance in subjects with NGT after treatment. However, at this stage of CFRD, very early structural alterations of islets can be observed in the pancreata of CF patients. As such, CFRD suggests that the CFTR modulator is of low interest at this stage of the disease. Bogdani et al. [
2] have evaluated the morphology of tissue from very young CF children (<4 years of age), as well as adult patients with CF and CFRD. The relative number of beta cells in young CF tissue was reduced by 50% or more, when compared to age-matched controls. Furthermore, young CF tissue displayed significantly smaller insulin-positive areas. CFRD pancreata exhibited greater islet injury, with further reduction in islet density and a decreased relative number of beta cells. Together, these results strongly suggest that an early deficiency in beta-cell number in CF may contribute to the development of glucose intolerance in the young CF population and, later in life, to CFRD.
Although our analysis could not exclude any impact of the natural disease history, various longitudinal studies have reported spontaneous improvements in glucose tolerance in CF patients, as well as improvements in patients’ nutritional status and respiratory function. This is due to the natural disease history. To illustrate this point, Manfred Ballmann cited data from the Scheuing study [3], which observed substantial variability in glucose-tolerance abnormalities in CF, as a large cohort of 1128 CF patients benefited from 4643 OGTT over 9 years. Scheuing reported regression from CFRD to NGT in 21.7% of cases, which is comparable with our study data (22.2%, including 31 patients with IGT and 9 with CFRD). Interestingly, when we analyze Scheuing’s [3] data for patients with IGT, 40.1% of patients returned to NGT and 14.6% altered their glucose tolerance and developed a CFRD. Conversely, in our study, 58% of patients returned to NGT and no patients presented with diabetes. Obviously, analysis of these data should consider the limited number of patients involved in our study.
In comparison with other types of diabetes, CFRD is characterized by very particular abnormalities of glucose tolerance. Early postprandial hyperglycemia is indicated by one-hour glucose values at OGTT and by continuous glucose measurement, which may impact lung function and nutritional status for several years prior to the development of diabetes. Recently, research has highlighted a decrease in the incretin effect, together with the role of a CFTR chloride-channel defect, in terms of both beta-cell function and also in alpha cells. This facilitates better understanding of these particular glucose-tolerance abnormalities, as impaired suppressibility of the glucagon release has been reported in CF patients, after an OGTT that possibly contributes to the development of glucose intolerance [4]. From our perspective, it is impossible for our study to exclude the influence of the lumacaftor–ivacaftor on the improvement of glucose tolerance.
However, our study suggests that the CFTR modulator plays a positive role at the very early stage of glucose-tolerance abnormalities in CF, without being able to demonstrate whether this is a direct effect that targets CFTR, the consequence of an improvement in nutritional and respiratory status, or both. Currently, there is an overall lack of large studies that explore treatments for CFRD. As such, there is a need for more analysis of metabolic parameters in randomized studies that evaluate more effective CFTR modulators (e.g., the new triple-combination CFTR modulator [5].
- Misgault, B., et al., Effect of one-year lumacaftor-ivacaftor treatment on glucose tolerance abnormalities in cystic fibrosis patients. J Cystic Fibrosis.https://doi.org/10.1016/j.jcf.2020.03.002. (Abstract included in section 2020B of cysticfibrosis.online)
- Bogdani M.et alStructural abnormalities in islets from very young children with cystic fibrosis may contribute to cystic fibrosis-related diabetes 2017; 7: 17231https://doi.org/10.1038/s41598-017-17404-z
- Scheuing N.et al.High variability in oral glucose tolerance among 1,128 patients with cystic fibrosis: a multicenter screening study.PLoS ONE. 2014; 9e112578
- Edlund A.et al.CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells.Sci Rep. 2017; 7: 90https://doi.org/10.1038/s41598-017-00098-8
- Middleton P.G.et al.Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019; 381: 1809-1819 32387043
Laurence Kessler is Professor of Endocrinology and Diabetology at Strasbourg University Hospitals, France
Laselva O, Bartlett C, Popa A, Ouyang H, Gunawardena TNA, Gonska T, Moraes TJ, Bear CE. Emerging preclinical modulators developed for F508del-CFTR have the potential to be effective for ORKAMBI resistant processing mutants. J Cyst Fibros 2020 Jul 30;S1569-1993(20)30807-9. doi: 10.1016/j.jcf.2020.07.015.Online ahead of print. [Pubmed]

Onofrio Laselva
F508del is prototypical of Class 2 CFTR mutations associated with protein misprocessing and reduced function. Corrector compounds like lumacaftor partially rescue the processing defect of F508del-CFTR whereas potentiators like ivacaftor, enhance its channel activity once trafficked to the cell surface. We asked if emerging modulators developed for F508del-CFTR can rescue Class 2 mutations previously shown to be poorly responsive to lumacaftor and ivacaftor.
Methods: Rescue of mutant CFTRs by the correctors: AC1, AC2-1 or AC2-2 and the potentiator, AP2, was studied in HEK-293 cells and in primary human nasal epithelial (HNE) cultures, using a membrane potential assay and Ussing chamber, respectively.
Results: In HEK-293 cells, we found that a particular combination of corrector molecules (AC1 plus AC2-1) and a potentiator (AP2) was effective in rescuing both the misprocessing and reduced function of M1101K and G85E respectively. These findings were recapitulated in patient-derived nasal cultures, although another corrector combination, AC1 plus AC2-2 also improved misprocessing in these primary tissues. Interestingly, while this corrector combination only led to a modest increase in the abundance of mature N1303K-CFTR it did enable its functional expression in the presence of the potentiator, AP2, in part, because the nominal corrector, AC2-2 also exhibits potentiator activity.
Conclusions: Strategic combinations of novel modulators can potentially rescue Class 2 mutants thought to be relatively unresponsive to lumacaftor and ivacaftor.
Dr Onofrio Laselva is with the Programme in Molecular Medicine, Hospital for Sick Children, Toronto, Canada; Department of Physiology, University of Toronto, Toronto, Canada
Lopes-Pacheco M CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine, Front Pharmacol. 2020 Feb 21;10:1662. doi: 10.3389/fphar.2019.01662. eCollection 2019. [Pubmed] (Full version on internet)

Miquelas Lopez-pacheco
Cystic fibrosis (CF) is a lethal inherited disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which result in impairment of CFTR mRNA and protein expression, function, stability or a combination of these. Although CF leads to multifaceted clinical manifestations, the respiratory disorder represents the major cause of morbidity and mortality of these patients. The life expectancy of CF patients has substantially lengthened due to early diagnosis and improvements in symptomatic therapeutic regimens. Quality of life remains nevertheless limited, as these individuals are subjected to considerable clinical, psychosocial and economic burdens. Since the discovery of the CFTR gene in 1989, tremendous efforts have been made to develop therapies acting more upstream on the pathogenesis cascade, thereby overcoming the underlying dysfunctions caused by CFTR mutations. In this line, the advances in cell-based high-throughput screenings have been facilitating the fast-tracking of CFTR modulators. These modulator drugs have the ability to enhance or even restore the functional expression of specific CF-causing mutations, and they have been classified into five main groups depending on their effects on CFTR mutations: potentiators, correctors, stabilizers, read-through agents, and amplifiers. To date, four CFTR modulators have reached the market, and these pharmaceutical therapies are transforming patients’ lives with short- and long-term improvements in clinical outcomes. Such breakthroughs have paved the way for the development of novel CFTR modulators, which are currently under experimental and clinical investigations. Furthermore, recent insights into the CFTR structure will be useful for the rational design of next-generation modulator drugs. This review aims to provide a summary of recent developments in CFTR-directed therapeutics. Barriers and future directions are also discussed in order to optimize treatment adherence, identify feasible and sustainable solutions for equitable access to these therapies, and continue to expand the pipeline of novel modulators that may result in effective precision medicine for all individuals with CF
Marcus A Mall ENaC inhibition in cystic fibrosis: potential role in the new era of CFTR modulator therapies. Eur Respir J 2020 Jul 30;2000946.doi: 10.1183/13993003.00946-2020.Online ahead of print [Pubmed]

Marcus A Mall
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators are the first approved drugs targeting underlying epithelial ion/fluid transport defects in patients with cystic fibrosis (CF). Current CFTR modulators restore mutant CFTR activity to up to ∼50% of normal CFTR Cl– channel function, translating into improvements in percentage predicted FEV1 and other clinical outcomes. In addition, reductions in airway bacterial colonisation are observed; however, patients fail to eradicate bacteria over time and still experience pulmonary exacerbations, and long-term safety of CFTR modulator therapy remains unknown. Currently approved CFTR modulators are predicted to be effective for up to 90% of patients. A mutation-agnostic approach could address the remaining 10% with CFTR mutations unresponsive to CFTR modulator therapy and may act together with CFTR modulator therapy to further improve epithelial ion/fluid transport and clinical outcomes. Together with CFTR and other Cl– channels, the epithelial Na+ channel (ENaC) is key to regulating airway surface liquid homeostasis. ENaC activity is limiting for Na+/fluid absorption and remains intact or may even be increased in CF airways, leading to increased Na+/fluid absorption, airway surface dehydration, impaired mucociliary clearance, bacterial infection, inflammation and progressive lung damage – the major cause of CF-related morbidity and mortality. Inhibition of ENaC in the airways is therefore an attractive therapeutic target to counteract airway surface dehydration and downstream consequences in CF lung disease. This review examines ENaC inhibition in CF therapy, and describes a new ENaC inhibitor with potential mutation-agnostic therapeutic benefit, both alone, and in synergy with CFTR modulators.
Professor Marcus Mall is at Department of Pediatric Pulmonology, Immunology and Critical Care Medicine, Charité – Universitätsmedizin Berlin, Berlin Institute of Health and the German Center for Lung Research (DZL), associated partner site, Berlin.
Mayer-Hamblett N, van Koningsbruggen-Rietschel S, Nichols DP, VanDevanter DR, Davies JC, Lee T, Durmowicz AG, Ratjen F, Konstan MW, Pearson K, Bell SC, Clancy JP, Taylor-Cousar JL, De Boeck K, Donaldson SH, Downey DG, Flume PA, Drevinek P, Goss CH, Fajac I, Magaret AS, Quon BS, Singleton SM, VanDalfsen JM, Retsch-Bogart GZ. Building global development strategies for cf therapeutics during a transitional cftr modulator era. J Cyst Fibros. 2020 Jun 7:S1569-1993(20)30161-2. doi:10.1016/j.jcf.2020.05.011. Online ahead of print. [Pubmed]

Nicole Mayer-Hamblett
As CFTR modulator therapy transforms the landscape of cystic fibrosis (CF) care, its lack of uniform access across the globe combined with the shift towards a new standard of care creates unique challenges for the development of future CF therapies. The advancement of a full and promising CF therapeutics pipeline remains a necessary priority to ensure maximal clinical benefits for all people with CF. It is through collaboration across the global CF community that we can optimize the evaluation and approval process of new therapies. To this end, we must identify areas for which harmonization is lacking and for which efficiencies can be gained to promote ethical, feasible, and credible study designs amidst the changing CF care landscape. This article summarizes the counsel from core advisors across multiple international regions and clinical trial networks, developed during a one-day workshop in October 2019. The goal of the workshop was to identify, in consideration of the highly transitional era of CFTR modulator availability, the drug development areas for which global alignment is currently uncertain, and paths forward that will enable advancement of CF therapeutic development.
Nicole Hamblett is in the University of Washington, Seattle, WA; Seattle Children’s Hospital, Seattle, WA. Electronic address: nicole.hamblett@seattlechildrens.org.
Dr Miqueias Lopes-Pacheco is at Biosystems & Integrative Sciences Institute, Faculty of Sciences, University of Lisbon, Lisbon, Portugal.
– This is a very detailed extensively referenced account of the present situation concerning modulators. The full text is available and can be recommended.
Misgault B, Chatron E, Reynaud Q, Touzet S, Abely M, Melly L, Dominique S, Troussier F, Ronsin-Pradel O, Gerardin M, Mankikian J, Cosson L, Chiron R, Bounyar L, Porzio M, Durieu I, Weiss L, Kessler R, Kessler L. Effect of one-year lumacaftor-ivacaftor treatment on glucose tolerance abnormalities in cystic fibrosis patients. J Cyst Fibros. 2020 Mar 19. pii: S1569-1993(20)30073-4. doi: 10.1016/j.jcf.2020.03.002. [Epub ahead of print] [Pubmed
To investigate the effects of 1-year lumacaftor-ivacaftor treatment on abnormalities in glucose tolerance (AGT) in Phe508del homozygous cystic fibrosis (CF) patients. Untreated CF patients with glucose intolerance or newly diagnosed diabetes were included in a prospective, observational study. After 1-year lumacaftor-ivacaftor treatment, AGT were evaluated by using oral glucose tolerance test.
Forty patients participated. 78% of patients had glucose intolerance and 22% diabetes at baseline. After one-year treatment, 50% of patients had normal glucose tolerance, 40% glucose intolerance, and 10% diabetes (p <0.001). The two-hour OGTT glycemia decreased from 171 (153-197) to 139 (117-162) mg/dL (p <0.001). 57.5% (n = 23) of patients improved their glucose tolerance with a significant decrease in both 1-hour (p<0.01) and 2-hour (p<0.001) OGTT glycemia.
The authors concluded improvements in AGT were observed following 1-year lumacaftor-ivacaftor treatment. Larger studies are needed to comprehensively assess CF transmembrane conductance regulator (CFTR) modulators.
Dr B Misgault is at the Service d’endocrinologie, diabète et nutrition, Hôpitaux Universitaires de Strasbourg, place de l’hôpital, Strasbourg 67091, France
Munck A, Kerem E, Ellemunter H, Campbell D, Wang LT, Ahluwalia N, Owen CA, Wainwright C. Tezacaftor/ivacaftor in people with cystic fibrosis heterozygous for minimal function CFTR mutations. J Cyst Fibros. 2020 Jun 13:S1569-1993(20)30128-4. doi: 10.1016/j.jcf.2020.04.015. Online ahead of print. [Pubmed]

Anne Munck
Tezacaftor/ivacaftor is a CFTR modulator approved to treat people with cystic fibrosis (pwCF) who are homozygous (F/F) or heterozygous for the F508del-CFTR mutation and a residual function mutation (F/RF).
This randomized, double-blind, placebo-controlled Phase 3 study evaluated the efficacy, safety, tolerability, and pharmacokinetics (PK) of tezacaftor/ivacaftor in participants ≥12 years of age heterozygous for the F508del-CFTR mutation and a minimal function mutation (F/MF), which produces no CFTR protein or a protein unresponsive to tezacaftor/ivacaftor in vitro.
Participants were randomized 1:1 to receive tezacaftor/ivacaftor or placebo for 12 weeks. The primary endpoint was the absolute change from baseline in percent predicted forced expiratory volume in 1 second (ppFEV1) between the tezacaftor/ivacaftor and placebo groups through week 12. Key secondary endpoints included absolute change from baseline in CF Questionnaire-Revised respiratory domain scores and the number of pulmonary exacerbations through week 12 and the absolute change from baseline in body mass index at week 12. A prespecified interim analysis (IA) for futility was conducted when approximately 50% of a planned enrolment of 300 participants reached week 12 of the study.
At the time of the interim analysis 83 participants were randomized to tezacaftor/ivacaftor and 85 to placebo; 165 participants completed treatment. The study failed to demonstrate that tezacaftor/ivacaftor significantly improved ppFEV1 or any of the key secondary endpoints and was terminated for futility. The safety profile and PK parameters of tezacaftor/ivacaftor were similar to those reported in prior studies in participants ≥12 years of age with CF.
Conclusions: Tezacaftor/ivacaftor did not show a clinically meaningful benefit in participants with F/MF genotypes but was generally safe and well tolerated, consistent with the safety profile reported in other Phase 3 studies (NCT02516410).
Dr Anne Munck is at the Robert Debré Hospital, Assistance Publique-Hopitaux de Paris, Université Paris Diderot, Paris, France
Nash EF, Middleton PG, Taylor-Cousar JL. Outcomes of pregnancy in women with cystic fibrosis (CF) taking CFTR modulators – an international survey. J Cyst Fibros. 2020 Mar 6. pii: S1569-1993(20)30067-9. doi: 10.1016/j.jcf.2020.02.018. [Epub ahead of print] [Pubmed]

Edward Nash
A survey was sent to lead clinicians of adult CF centres in Europe, the United Kingdom (UK), United States of America (USA), Australia and Israel requesting anonymised data on pregnancy outcomes in women using CFTR modulators before and during pregnancy and lactation. The survey identified 64 pregnancies in 61 women taking IVA (n = 31), LUM/IVA (n = 26) or TEZ/IVA (n = 7), resulting in 60 live births. In 44 pregnancies, CFTR modulators were either continued throughout pregnancy or temporarily stopped and then restarted. Two maternal complications were deemed related to CFTR modulator therapy; cessation of modulator therapy resulted in clinical decline in 9 women prompting resumption of therapy during pregnancy. No modulator-related complications were reported in infants exposed in utero and/or during breastfeeding.
CFTR modulators were reported to be generally well tolerated in pregnancy and breastfeeding, with only 2 maternal complications that were deemed related to CFTR modulator therapy. Women stopping CFTR modulators in pregnancy may experience a decline in clinical status and in the cases identified in this survey, restarting therapy led to a clinical improvement. Current experience remains limited and longer-term prospective follow-up is required to exclude delayed adverse effects.
Dr Edward Nash is a Pulmonologist West Midlands Adult Cystic Fibrosis Centre, University Hospitals Birmingham NHS Foundation Trust, Birmingham
New Drug Hailed as Major Breakthrough in Cystic Fibrosis. Am J Med Genet A. 2020 Jan;182(1):8-9. doi: 10.1002/ajmg.a.61420.[Pubmed] My summary as their is no abstract: No authors are listed for this neat account of the progress in the development of CFTR modulator therapy from the FDA approval of ivacaftor (VX-771) in January 2012 for patients with one copy of the G551D mutation, to Orkambi, the combination of lumacaftor (VX-809) and ivacaftor, for those with 2 copies of the F508del mutation in 2015, to another drug combination of tezacaftor (VX-661) and ivacaftor (Symdeko) in 2018 for those people with at least one F508del mutation.
In 2019 the combination drug Trikafta, combining elexacaftor (VX-445), ivacaftor (Kalydeco) and tezacaftor (VX-661), was approved for patients aged 12 years and over with at least one F508del – a group comprising some 90% of the CF population. Approval was based on two phase III clinical trials (Middleton et al, 2019; Heijerman et al, 2019). Sadly these drugs are very expensive and funding is and will remain a major problem.
Nichols AL, Davies JC, Jones D, Carr SB. Restoration of exocrine pancreatic function in older children with cystic fibrosis on ivacaftor. Paediatr Respir Rev. 2020 Apr 14. pii: S1526-0542(20)30073-7. doi: 10.1016/j.prrv.2020.04.003. [Epub ahead of print] Review. [Pubmed]
Prior to the use of cystic fibrosis (CF) modulator therapy, exocrine pancreatic insufficiency in CF was thought to be irreversible. Improvement in pancreatic function on ivacaftor has been reported in open label studies in 1-5 year olds. The mechanism by which ivacaftor might improve exocrine pancreatic function is unclear. Although the effect of ivacaftor on pancreatic function may be more significant in younger children, evidence is mounting that there may still be potential for improvement in older children on long term therapy.
Dr A L Nichols is in the Department of Paediatric Respiratory Medicine, Royal Brompton and Harefield NHS Foundation Trust, London, UK.
Olivereau L, Nave V, Garcia S, Perceval M, Rabilloud M, Durieu I, Reynaud Q. Adherence to lumacaftor-ivacaftor therapy in patients with cystic fibrosis in France.
J Cyst Fibros. 2020 Jan 2. pii: S1569-1993(19)30901-4. doi: 10.1016/j.jcf.2019.09.018. [Epub ahead of print] [Pubmed]
This retrospective study used pharmacy refills data to calculate proportion of days covered (PDC). Adherence was defined as a PDC ≥80%. A logistic regression analysis was conducted to examine factors associated with medication adherence.
Ninety-six patients were included in the final cohort for analysis. The mean PDC was 96% ± 14 at 6 months, and 91% ± 17 at 12 months. The proportion of adherent patients was 89% and 83% at 6 and 12 months respectively. Age and ppFEV1 were found to affect medication adherence.
Considering the medico-economic impact of CFTR modulator therapy, high adherence rates to lumacaftor-ivacaftor found in this study are encouraging.
Dr L Olivereau is at the Pharmacie Centrale, Hospices Civils de Lyon, F-69230 Saint Genis Laval, France.
Perrem L, Ratjen F. Anti-inflammatories and mucociliary clearance therapies in the age of CFTR modulators. Pediatr Pulmonol. 2019;54 Suppl 3:S46‐S55. doi:10.1002/ppul.24364 [Pubmed]

Lucy Perrem
The combination of CFTR dysfunction, mucus obstruction, and infection drive an exaggerated and dysfunctional inflammatory response, which contributes to irreversible airway destruction and fibrosis. CFTR modulators, an exciting new class of drugs, increase the expression and/or function of CFTR variant protein and improve multiple clinical endpoints, such as lung function, pulmonary exacerbation rates, and nutritional status.
However, these genotype-specific drugs are not universally available, the clinical response is variable, and lung function still declines over time when bronchiectasis is established. Consequently, even in the age of CFTR modulators, we must target other important aspects of the CF airway disease, such as inflammation and mucociliary clearance. This review highlights the mechanisms of inflammation and mucus accumulation in the CF lung and discusses anti-inflammatory and mucociliary clearance agents that are currently in development focusing on compounds for which clinical trial data have recently become available.
Dr Lucy Perrem is in the Division of Respiratory Medicine, The Department of Paediatrics, The Hospital for Sick Children, Toronto, Canada.
Joseph M Pilewski , Kris De Boeck, Jerry A Nick, Simon Tian, Cynthia DeSouza, Mark Higgins, Richard B Moss. Long-Term Ivacaftor in People Aged 6 Years and Older with Cystic Fibrosis with Ivacaftor-Responsive Mutations. Pulm Ther . 2020 Sep 23. doi: 10.1007/s41030-020-00129-2. Online ahead of print. [Pubmed]

Joseph Pilewski
The study evaluated the long-term safety and efficacy of ivacaftor in people with CF aged 6 years and older with non-G551D-CFTR ivacaftor-responsive mutations. Efficacy and safety data from a phase 3, multicenter, open-label, extension study for participants from Study 110 (R117H-CFTR mutations), Study 111 (non-G551D-CFTR gating mutations), and Study 113 (n-of-1 pilot study in participants with residual CFTR function) were analyzed. Following washout from the randomized parent study, participants received oral ivacaftor 150 mg once every 12 h for 104 weeks.
Forty-one of 121 participants completed treatment through 104 weeks; 59 participants who did not complete the extension study continued treatment with commercial ivacaftor. The most common adverse events were pulmonary exacerbation (46.3%) and cough (33.9%). Most treatment-emergent adverse events were mild/moderate in severity and consistent with manifestations of CF or the ivacaftor safety profile. Rapid, durable improvement occurred across all efficacy endpoints.
Ivacaftor was generally safe and well tolerated with no new safety concerns for up to 104 weeks in people with CF with ivacaftor-responsive mutations. The pattern of improvement across efficacy endpoints was durable and generally consistent with parent-study outcomes. (NCT01707290).
Dr Joseph M Pilewski is Associate Chief, Division of Pulmonary, Allergy & Critical Care Medicine and Associate Professor of Medicine, Cell Biology, Physiology & Pediatrics, University of Pittsburgh,
Melissa S Putman, Logan B Greenblatt, Michael Bruce, Taisha Joseph, Hang Lee, Gregory Sawicki, Ahmet Uluer, Leonard Sicilian, Isabel Neuringer, Catherine M Gordon, Mary L Bouxsein, Joel S Finkelstein. The Effects of Ivacaftor on Bone Density and Microarchitecture in Children and Adults with Cystic Fibrosis. J Clin Endocrinol Metab. 2020 Dec 1:dgaa890. doi: 10.1210/clinem/dgaa890. Online ahead of print [Pubmed]

Melissa Putman
Context: Cystic fibrosis transmembrane conductance (CFTR) dysfunction may play a role in CF-related bone disease (CFBD). Ivacaftor is a CFTR potentiator effective in improving pulmonary and nutritional outcomes in patients with the G551D-CFTR mutation. The effects of ivacaftor on bone health are unknown.
Objective: To determine the impact of ivacaftor on bone density and microarchitecture in children and adults with CF.
Design: Prospective observational multiple cohort study.
Setting: Outpatient clinical research center within a tertiary academic medical center.
Patients or other participants: Three cohorts of age-, race-, and gender-matched subjects were enrolled: 26 subjects (15 adults and 11 children) with CF and the G551D-CFTR mutation who were planning to start or had started treatment with ivacaftor within three months (Ivacaftor cohort); 26 subjects with CF were not treated with ivacaftor (CF Control cohort); and 26 healthy volunteers.
Interventions: All treatments, including ivacaftor, were managed by the subjects’ pulmonologists.
Main outcome measures: Bone microarchitecture by high resolution peripheral quantitative computed tomography (HR-pQCT), areal bone mineral density (aBMD) by dual-energy X-ray absorptiometry (DXA) and bone turnover markers at baseline, 1, and 2 years.
Results: Cortical volume, area, and porosity at the radius and tibia increased significantly in adults in the Ivacaftor cohort. No significant differences were observed in changes in aBMD, trabecular microarchitecture, or estimated bone strength in adults or in any outcome measures in children.
Conclusions: Treatment with ivacaftor was associated with increases in cortical microarchitecture in adults with CF. Further studies are needed to understand the implications of these findings.
Dr Melissa S Putman is a paediatric and adult endocrinologist in the Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, MA. and the Division of Endocrinology, Boston Children’s Hospital, Boston, MA.
Fiona Qiu, Mark Habgood, Elena K Schneider-Futschik The Balance between the Safety of Mother, Fetus, and Newborn Undergoing Cystic Fibrosis Transmembrane Conductance Regulator Treatments during Pregnancy. ACS Pharmacol Transl Sci . 2020 Aug 19;3(5):835-843. doi: 10.1021/acsptsci.0c00098. eCollection 2020 Oct 9. [Pubmed]

Elena Schneider-Futschik
The recent development of modulators of cystic fibrosis transmembrane conductance regulator (CFTR) has allowed the life expectancy of cystic fibrosis patients to increase substantially resulting in more women with cystic fibrosis reaching child-bearing age. This however raises the issue of whether long-term use of CFTR modulators during pregnancy and breastfeeding is safe for the fetus and newborn, especially for their developing brain. A very limited number of case reports available so far has shown that the fetus or breastfed newborn is likely to be exposed to maternally administered CFTR modulators. Potential impacts of drug exposure on the developing brain are of particular importance as the consequences might not be immediately noticeable upon birth but may manifest later in life as permanent neurobehavioral problems. In order for drugs in maternal circulation to enter the fetal brain, they must overcome the placental barrier followed by a series of brain barriers, each consisting of cellular components and physiological mechanisms such as efflux transporters. The extent of protection they offer during development will provide valuable insights into the potential entry and the effects of CFTR modulators in the developing brain. This review aims to explore the current understanding of the safety of CFTR modulators, especially ivacaftor, during pregnancy and breastfeeding, characterize the pharmacokinetics and pharmacodynamics of ivacaftor, both under normal conditions and during pregnancy, to provide context for its potential impact on the developing brain. Finally, we discuss the determinants that need to be taken into consideration when investigating the entry of drugs into the fetus and newborn.
Fiona Qiu is at the Department of Pharmacology & Therapeutics, Lung Health Research Centre, School of Biomedical Sciences, Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Parkville, Victoria 3010, Australia.
Dr Elena K Schneider-Futschik is the senior research fellow at the Institute
Dr Lara Pullen is a medical writer who has covered a wide range of topics.
Rang C, Keating D, Wilson J, Kotsimbos T. Re-Imagining Cystic Fibrosis Care: Next Generation Thinking. Eur Respir J. 2020 Mar 5. pii: 1902443. doi: 10.1183/13993003.02443-2019. [Epub ahead of print][Pubmed]

Tom Kotsimbos
Cystic fibrosis is a common multi-system genetically inherited condition, predominately found in individuals of Caucasian decent. Since the identification of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in 1989, and the subsequent improvement in understanding of CF pathophysiology, significant increases in life-expectancy have followed. Initially this was related to improvements in the management and systems of care for treating the various affected organ systems. These cornerstone treatments are still essential for CF patients born today. However, over the last decade, the major advance has been in therapies that target the resultant genetic defect – the dysfunctional CFTR protein. Small molecule agents that target this dysfunctional protein via a variety of mechanisms have led to lung function improvements, reductions in pulmonary exacerbation rates and increases in weight and quality of life indices. As more patients receive these agents earlier and earlier in life, it is likely that general CF care will increasingly pivot around these specific therapies, although it is also likely that effects other than those identified in the initial trials will be discovered and need to be managed. Despite great excitement for modulator therapies, they are unlikely to be suitable or available for all: whether this is due to a lack of availability for specific CFTR mutations, drug-reactions or the health economic set-up in certain countries. Nevertheless, the CF community must be applauded for its ongoing focus on research and development for this life-limiting disease. With time, personalised individualised therapy would ideally be the mainstay of CF care.
– This is a clear summary of the future changing nature of CF care. As with many other such articles there is no mention of prevention.
Dr Tom Kotsimbos is Associate Professor and Head of Respiratory Medicine Laboratory, AIRMed Department, Central Clinical School, Monash University.
Giada Righetti, Monica Casale, Michele Tonelli, Nara Liessi , Paola Fossa, Nicoletta Pedemonte, Enrico Millo , Elena Cichero. New Insights into the Binding Features of F508del CFTR Potentiators: A Molecular Docking, Pharmacophore Mapping and QSAR Analysis Approach. Pharmaceuticals (Basel) 2020 Dec 4;13(12):E445.doi: 10.3390/ph13120445. [Pubmed]

Giada Righetti
Cystic fibrosis (CF) is the autosomal recessive disorder most recurrent in Caucasian populations. To combat this disease, many life-prolonging therapies are required and deeply investigated, including the development of the so-called cystic fibrosis transmembrane conductance regulator (CFTR) modulators, such as correctors and potentiators. Combination therapy with the two series of drugs led to the approval of several multi-drug effective treatments, such as Orkambi, and to the recent promising evaluation of the triple-combination Elexacaftor-Tezacaftor-Ivacaftor.
This scenario enlightened the effectiveness of the multi-drug approach to pave the way for the discovery of novel therapeutic agents to contrast CF. The recent X-crystallographic data about the human CFTR in complex with the well-known potentiator Ivacaftor (VX-770) opened the possibility to apply a computational study aimed to explore the key features involved in the potentiator binding.
Herein, we discussed molecular docking studies performed onto the chemotypes so far discussed in the literature as CFTR potentiator, reporting the most relevant interactions responsible for their mechanism of action, involving Van der Waals interactions and π-π stacking with F236, Y304, F305 and F312, as well as H-bonding F931, Y304, S308 and R933.
This kind of positioning will stabilize the effective potentiator at the CFTR channel. These data have been accompanied by pharmacophore analyses, which promoted the design of novel derivatives endowed with a main (hetero)aromatic core connected to proper substituents, featuring H-bonding moieties. A highly predictive quantitative-structure activity relationship (QSAR) model has been developed, giving a cross-validated r2 (r2cv) = 0.74, a non-cross validated r2 (r2ncv) = 0.90, root mean square error (RMSE) = 0.347, and a test set r2 (r2pred) = 0.86.
On the whole, the results are expected to gain useful information to guide the further development and optimization of new CFTR potentiators
Giada Righetti is a PhD student in the Department of Pharmacy, Section of Medicinal Chemistry, School of Medical and Pharmaceutical Sciences, University of Genoa, Viale Benedetto XV 3, 16132 Genoa, Italy.
Geraint B Rogers, Steven L Taylor, Lucas R Hoffman, Lucy D Burr. The impact of CFTR modulator therapies on CF airway microbiology. J Cyst Fibros 2020 May;19(3):359-364. doi: 10.1016/j.jcf.2019.07.008.Epub 2019 Aug [Pubmed]

Geraint Rodgers
Major historical advances in cystic fibrosis (CF) respiratory clinical care, including mechanical airway clearance and inhaled medications, have aimed to address the consequences of cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. In contrast, CFTR modulator therapies instead target the underlying protein defect that leads to CF lung disease. The extent to which these therapies might reduce susceptibility to chronic lung infections remains to be seen. However, by improving airway clearance, reducing the requirement for antibiotics, and in some cases, through direct antimicrobial effects, CFTR modulators are likely to result in substantial changes in CF airway microbiology. These changes could contribute substantially to the clinical benefit associated with modulator therapies, as well as providing an important indicator of treatment efficacy and residual pathophysiology. Indeed, the widespread introduction of modulator therapies might require us to re-consider our models of CF airway microbiolo
Prof Geraint B Rogers is a molecular microbiologist and microbial ecologist. He is the Director, Microbiome Research at SAHMRI, leads a laboratory based within the Flinders University School of Medicine, Adelaide
Steven M Rowe, Ieuan Jones Mark T Dransfield, Nazmul Haque, Stephen Gleason , Katy A Hayes , Kenneth Kulmatycki , Denise P Yates, Henry Danahay, Martin Gosling , David J Rowlands, Sarah S Grant Efficacy and Safety of the CFTR Potentiator Icenticaftor (QBW251) in COPD: Results from a Phase 2 Randomized Trial Int J Chron Obstruct Pulmon Dis 2020 Oct 5;15:2399-2409.doi: 10.2147/COPD.S257474. eCollection 2020. Free PMC article [Pubmed]

Steven Rowe
Rationale: Excess mucus plays a key role in COPD pathogenesis. Cigarette smoke-induced cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction may contribute to disease pathogenesis by depleting airway surface liquid and reducing mucociliary transport; these defects can be corrected in vitro by potentiating CFTR.
Objective: To assess the efficacy of the CFTR potentiator icenticaftor in improving airflow obstruction in COPD patients with symptoms of chronic bronchitis.
Methods: In this double-blind, placebo-controlled study, COPD patients were randomized (2:1) to either icenticaftor 300 mg or placebo b.i.d. This non-confirmatory proof of concept study was powered for lung clearance index (LCI) and pre-bronchodilator FEV1, with an estimated sample size of 90 patients. The primary endpoint was change from baseline in LCI for icenticaftor versus placebo at Day 29; key secondary endpoints included change from baseline in pre- and post-bronchodilator FEV1 on Day 29. Key exploratory endpoints included change from baseline in sweat chloride, plasma fibrinogen levels, and sputum colonization.
Results: Ninety-two patients were randomized (icenticaftor, n=64; placebo, n=28). At Day 29, icenticaftor showed no improvement in change in LCI (treatment difference: 0.28 [19% probability of being better than placebo]), an improvement in pre-bronchodilator FEV1 (mean: 50 mL [84% probability]) and an improvement in post-bronchodilator FEV1 (mean: 63 mL [91% probability]) over placebo. Improvements in sweat chloride, fibrinogen and sputum bacterial colonization were also observed. Icenticaftor was safe and well tolerated.
Conclusion: The CFTR potentiator icenticaftor increased FEV1 versus placebo after 28 days and was associated with improvements in systemic inflammation and sputum bacterial colonization in COPD patients; no improvements in LCI with icenticaftor were observed.
Dr Steven M Rowe is Professor and Director, Cystic Fibrosis Research Centeris at the University of Alabama at Birmingham, Department of Medicine, Birmingham, AL, USA.
Afsoon Sepahzad, Deborah J Morris-Rosendahl, JaneDavies. Cystic Fibrosis Lung Disease Modifiers and Their Relevance in the New Era of Precision Medicine. Genes (Basel) 2021 Apr 13;12(4):562.doi: 10.3390/genes12040562. Free PMC article[Pubmed]
Our understanding of cystic fibrosis (CF) has grown exponentially since the discovery of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in 1989. With evolving genetic and genomic tools, we have come to better understand the role of CFTR genotypes in the pathophysiology of the disease. This, in turn, has paved the way for the development of modulator therapies targeted at mutations in the CFTR, which are arguably one of the greatest advances in the treatment of CF. These modulator therapies, however, do not target all the mutations in CFTRthat are seen in patients with CF and, furthermore, a variation in response is seen in patients with the same genotype who are taking modulator therapies. There is growing evidence to support the role of non-CFTR modifiers, both genetic and environmental, in determining the variation seen in CF morbidity and mortality and also in the response to existing therapies.
This review focusses on key findings from studies using candidate gene and genome-wide approaches to identify CF modifier genes of lung disease in cystic fibrosis and considers the interaction between modifiers and the response to modulator therapies. As the use of modulator therapies expands and we gain data around outcomes, it will be of great interest to investigate this interaction further. Going forward, it will also be crucial to better understand the relative influence of genomic versus environmental factors. With this understanding, we can truly begin to deliver personalised care by better profiling the likely disease phenotype for each patient and their response to treatment.
Dr Afsoon Sepahzad is in the Department of Paediatric Respiratory Medicine, Royal Brompton and Harefield Hospitals, London SW3 6NP, UK.
Shaw M, Khan U, Clancy JP, Donaldson SH, Sagel SD, Rowe SM, Ratjen F; PROSPECT Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Changes in LCI in F508del/F508del patients treated with lumacaftor/ivacaftor: Results from the prospect study. J Cyst Fibros. 2020 Jun 6:S1569-1993(20)30160-0. doi: 10.1016/j.jcf.2020.05.010. Online ahead of print. [Pubmed]
The PROSPECT study, a post-approval observational study in the U.S., showed no significant changes in lung function as measured by spirometry with clinical initiation of lumacaftor/ivacaftor. A sub-study within the PROSPECT study assessed the lung clearance index (LCI), as measured by multiple breath washout (MBW), a measure of lung function demonstrated to be sensitive among people with normal spirometry. Participants performed MBW prior to clinically initiating lumacaftor/ivacaftor therapy and for one year of follow-up. Similar to the whole PROSPECT study, this sub-study cohort (N = 49) had no significant absolute or relative changes in FEV1% predicted at any time point. LCI, however, decreased (improved) by 0.81 units or 5.3% (95% CI -9.7, -0.9%) at 1 month, 0.77 units or 5.9% at 3 months, 0.67 units or 5.9% at 6 months, and 0.55 units or 4.3% at 12 months. These results demonstrate the utility of the LCI in assessing treatment effects of relatively modest size in a heterogenous study population.
Department of Pediatrics, Hospital for Sick Children, Toronto, ON, Canada.
Ranjani Somayaji , Dave Nichols, Scott C Bell Cystic fibrosis – Ten promising therapeutic approaches in the current era of care. Expert Opin Investig Drugs. 2020 Aug 3.doi: 10.1080/13543784.2020.1805733.Online ahead of print. [Pubmed]
Introduction: Cystic fibrosis (CF) is a genetic disease affecting multiple organ systems. Research and innovations in novel therapeutic agents and health care delivery have resulted in dramatic improvements in quality of life and survival for people with CF. Despite this, significant disease burden persists for many and this is compounded by disparities in treatment access and care which globally necessitates further work to improve outcomes. Because of the advent of a numerous therapies which includes gene-targeted modulators in parallel with specialized care delivery models, innovative efforts continue.
Areas covered: In this review, the authors discuss the available data on investigational agents in clinical development and currently available treatments for CF. They also evaluate approaches to care delivery, consider treatment gaps and propose future directions for advancement.
Expert opinion: Since the discovery of the CF gene, CFTR modulators have provided a hallmark of success, even though it was thought not previously possible. This has led to reinvigorated efforts and innovations in treatment approaches and care delivery. Numerous challenges remain because of genetic and phenotypic heterogeneity, access issues, and therapeutic costs, but the collaborative approach between stakeholders for continued innovation fuels optimism.
Dr Ranjani Somayaji is Assistant Professor in the University of Calgary, Departments of Medicine | Microbiology, Immunology and Infectious Disease | Community Health Sciences. Institutional affiliations: Snyder Institute for Chronic Diseases | O’Brien Institute for Public Health
Jyoti Sharma, Kim M Keeling, Steven M Rowe. Pharmacological approaches for targeting cystic fibrosis nonsense mutations. Eur J Med Chem. 2020 Aug 15;200:112436.doi: 10.1016/j.ejmech.2020.112436.Epub 2020 May 21. Free PMC article [Pubmed]

Joyti Sharma
Cystic fibrosis (CF) is a monogenic autosomal recessive disorder. The clinical manifestations of the disease are caused by ∼2,000 mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It is unlikely that any one approach will be efficient in correcting all defects. The recent approvals of ivacaftor, lumacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor represent the genesis of a new era of precision combination medicine for the CF patient population. In this review, we discuss targeted translational readthrough approaches as mono and combination therapies for CFTR nonsense mutations. We examine the current status of efficacy of translational readthrough/nonsense suppression therapies and their limitations, including non-native amino acid incorporation at PTCs and nonsense-mediated mRNA decay (NMD), along with approaches to tackle these limitations. We further elaborate on combining various therapies such as readthrough agents, NMD inhibitors, and corrector/potentiators to improve the efficacy and safety of suppression therapy. These mutation specific strategies that are directed towards the basic CF defects should positively impact CF patients bearing nonsense mutations.
Jyoti Sharma is in the Department of Medicine, University of Alabama at Birmingham (UAB), USA; Department of Gregory Fleming James Cystic Fibrosis Research Center, University of Alabama at Birmingham (UAB), USA
Dr Zhuqing Shi is from the Program for Personalized Cancer Care, NorthShore University Health System, Evanston, IL.
Shaw M, Khan U, Clancy JP, Donaldson SH, Sagel SD, Rowe SM, Ratjen F; PROSPECT Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Changes in LCI in F508del/F508del patients treated with lumacaftor/ivacaftor: Results from the prospect study. J Cyst Fibros. 2020 Jun 6:S1569-1993(20)30160-0. doi: 10.1016/j.jcf.2020.05.010. Online ahead of print. [Pubmed]
The PROSPECT study, a post-approval observational study in the U.S., showed no significant changes in lung function as measured by spirometry with clinical initiation of lumacaftor/ivacaftor. A sub-study within the PROSPECT study assessed the lung clearance index (LCI), as measured by multiple breath washout (MBW), a measure of lung function demonstrated to be sensitive among people with normal spirometry. Participants performed MBW prior to clinically initiating lumacaftor/ivacaftor therapy and for one year of follow-up. Similar to the whole PROSPECT study, this sub-study cohort (N = 49) had no significant absolute or relative changes in FEV1% predicted at any time point. LCI, however, decreased (improved) by 0.81 units or 5.3% (95% CI -9.7, -0.9%) at 1 month, 0.77 units or 5.9% at 3 months, 0.67 units or 5.9% at 6 months, and 0.55 units or 4.3% at 12 months. These results demonstrate the utility of the LCI in assessing treatment effects of relatively modest size in a heterogenous study population.
Dr M Shaw Department of Pediatrics, Hospital for Sick Children, Toronto, ON, Canada.
Schwarz C, Sutharsan S, Epaud R, Klingsberg RC, Fischer R, Rowe SM, Audhya PK, Ahluwalia N, You X, Ferro TJ, Duncan ME, Bruinsma BG. Tezacaftor/ivacaftor in people with cystic fibrosis who stopped lumacaftor/ivacaftor due to respiratory adverse events [published online ahead of print, 2020 Jun 22]. J Cyst Fibros. 2020;S1569-1993(20)30730-X. doi:10.1016/j.jcf.2020.06.001

Carsten Schwarz
Increased rates of respiratory adverse events have been observed in people ≥12 years of age with cystic fibrosis homozygous for the Phe508del-CFTR mutation treated with lumacaftor/ivacaftor, particularly in those with percent predicted forced expiratory volume in 1 s (ppFEV1) of <40%. The safety, tolerability, and efficacy of tezacaftor/ivacaftor were evaluated in people with cystic fibrosis homozygous for Phe508del-CFTR who discontinued lumacaftor/ivacaftor due to treatment-related respiratory signs or symptoms.
Participants ≥12 years of age with cystic fibrosis homozygous for Phe508del-CFTR with ppFEV1 of ≥25% and ≤90% were randomized 1:1 and treated with tezacaftor/ivacaftor or placebo for 56 days.
Of 97 participants, 94 (96.9%) completed the study. The primary endpoint was incidence of predefined respiratory adverse events of special interest (chest discomfort, dyspnoea, respiration abnormal, asthma, bronchial hyperreactivity, bronchospasm, and wheezing): tezacaftor/ivacaftor, 14.0%; placebo, 21.3%. The adverse events were mild or moderate in severity. None were serious or led to treatment interruption or discontinuation. Overall, the discontinuation rate was similar between groups. The mean (SD) ppFEV1 at baseline was 44.6% (16.1%) with tezacaftor/ivacaftor and 48.0% (18.1%) with placebo. The posterior mean difference in absolute change in ppFEV1 from baseline to the average value of days 28 and 56 was 2.7 percentage points with tezacaftor/ivacaftor vs placebo.
The authors concluded tezacaftor/ivacaftor was generally safe, well tolerated, and efficacious in people ≥12 years of age with cystic fibrosis homozygous for Phe508del-CFTR with ppFEV1 of ≥25% and ≤90% who previously discontinued lumacaftor/ivacaftor due to treatment-related respiratory signs or symptoms.
Dr Carsten Schwarz at the Christiane Herzog Zentrum Berlin/Charité-Universitätsmedizin Berlin, Berlin, Germany.
Smyth RL. New drug treatments for cystic fibrosis. BMJ. 2020 Jan 20;368:m118. doi: 10.1136/bmj.m11 [Pubmed]

Rosiland Smyth
The present situation regarding the new CFTR modulators is neatly summarised by Prof. Ros Smyth in this BMJ article. As some readers may not have access to the full text, I have summarised some of the key points and added the key references –
Mutations that result in CFTR being expressed on the cell surface but incorrectly regulated, such as G551D, were the most straightforward targets. Ivacaftor, a potentiator, increases the time that the CFTR chloride channel remains open. In a phase III randomised placebo controlled trial, ivacaftor improved forced expiratory volume in one second (FEV1) by 11% as well as respiratory symptoms and weight gain without substantial adverse effects (Ramsey et al 2011). The trial recruited 161 patients and was completed in 19 months despite the small pool of just 2000 people worldwide with the G551D mutation. Ivacaftor was licensed in Europe in 2012.
Ivacaftor is effective for only 4-5% of patients with cystic fibrosis. Many more patients have the F508del mutation, which causes misfolding of CFTR protein so that it remains trapped in the cell’s endoplasmic reticulum. Any CFTR that does reach the cell surface is unable to activate normally. Although potentiators can enhance activation at the cell surface, dealing with the trapped protein requires agents that correct the misfolding (correctors).
In 2015, a trial of Orkambi, a drug combining the corrector lumacaftor with the potentiator ivacaftor, showed improvements in weight gain and respiratory exacerbations among patients who were homozygous for F508del, but the modest 3% increase in FEV1 relative to placebo was associated with transient dyspnoea and abnormal liver function (Wainwright et al, 2015)
Subsequent trials of a different corrector, tezacaftor, combined with ivacaftor showed similar improvements in FEV1 to Orkambi but with fewer side effects (Taylor-Cousar et al, 2017; Rowe SM et al, 2017)
In 2018, tezacaftor-ivacaftor (Symkevi) was licensed in the US and Europe for patients with F508del (either homozygous or F508del plus an allele with another mutation associated with residual CFTR function), representing around 50% of patients worldwide (Davies JC et al, 2018; Keating D et al, 2018).
Although 90% of people with cystic fibrosis have at least one copy of the F508del mutation, around 30% also have other minimal function mutations that are unresponsive to current CFTR modulators. This led to the development of next generation correctors and trials of “triple therapy” combining two correctors and a potentiator.
In 2019, phase III trials of elexacaftor-ivacaftor-tezacaftor in patients with F508del reported greater than 10% improvements in FEV1 compared with placebo and substantial reductions in respiratory exacerbations (Heijerman et al, 2019; Middleton et al, 2019).
– Ramsey BW, Davies J, McElvaney NG, et al., VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72. doi:10.1056/NEJMoa1105185 [Pubmed] –Wainwright CE, Elborn JS, Ramsey BW, et al., TRAFFIC Study Group, TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med2015;373:2231.doi:10.1056/NEJMoa1409547 [Pubmed]
– Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med2017;377:2013-23. doi:10.1056/NEJMoa1709846 [Pubmed]
– Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med2017;377:2024-35. doi:10.1056/NEJMoa1709847 [Pubmed]
– Davies JC, Moskowitz SM, Brown C, et al., VX16-659-101 Study Group. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med2018; 379:1599-611. doi:10.1056/NEJMoa1807119 pmid [Pubmed
– Keating D, Marigowda G, Burr L, et al., VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med2018; 379:1612-20. doi:10.1056/NEJMoa1807120 pmidL [Pubmed]
– Heijerman HGM, McKone EF, Downey DG, et al., VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet2019; 394:1940-8. doi:10.1016/S0140-6736(19)32597-8 [Pubmed]
– Middleton PG, Mall MA, Dřevínek P, et al., VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381:1809-19. doi:10.1056/NEJMoa1908639 [Pubmed]
The authors concluded Lumacaftor-ivacaftor was associated with improvement in lung disease and nutritional status in patients who tolerated treatment. Adults who discontinued lumacaftor-ivacaftor, often owing to adverse events, were found at high risk of clinical deterioration.
Shteinberg M, Taylor-Cousar JL. Impact of CFTR modulator use on outcomes in people with severe cystic fibrosis lung disease. Eur Respir Rev. 2020 Mar 20;29(155). pii: 190112. doi: 10.1183/16000617.0112-2019. Print 2020 Mar 31. Free full text [Pubmed]

Michal Shteinberg
Drug compounds that augment the production and activity of the cystic fibrosis (CF) transmembrane regulator (CFTR) have revolutionised CF care. Many adults and some children with CF suffer advanced and severe lung disease or await lung transplantation. While the hope is that these drug compounds will prevent lung damage when started early in life, there is an ongoing need to care for people with advanced lung disease. The focus of this review is the accumulating data from clinical trials and case series regarding the benefits of CFTR modulator therapy in people with advanced pulmonary disease. We address the impact of treatment with ivacaftor, lumacaftor/ivacaftor, tezacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor on lung function, pulmonary exacerbations, nutrition and quality of life. Adverse events of the different CFTR modulators, as well as the potential for drug-drug interactions, are discussed.
Dr Michal Shteinberg is heading the bronchiectasis and adult CF service in the Pulmonology institute and CF Center, Carmel medical Center, Haifa, Israel and a Clinical Assistant Professor in the Faculty of medicine at the Technion, Israel Institute of Technology.
Professor Stuart Elborn comments on this article as follows –
Elborn JS. Modulator treatment for people with cystic fibrosis: moving in the right direction. Eur Respir Rev. 2020 Mar 20;29(155). pii: 200051. doi: 10.1183/16000617.0051-2020. Print 2020 Mar 31. Free full text [Pubmed]

Stuart Elborn
Stuart Elborn in this issue of the European Respiratory Review, describes Shteinberg and Taylor-Cousar’s article as providing a very useful review of the current state of play in Europe for CFTR modulators
There is a benefit from these therapies even in individuals with severely reduced FEV1 who are excluded from the trials in follow-up clinical studies, and people with CF benefit from a significant reduction in pulmonary exacerbations. The adverse events and drug/drug interactions are comprehensively covered in the article by Shteinberg and Taylor-Cousar. Drug/drug interactions are also important in this group of drugs as they interact with a number of hepatic enzymes important in drug metabolism. These are particularly relevant for antibacterial and antifungal agents, which are often used in people with CF.
Elborn suggests the delivery of healthcare services for people with CF is likely to evolve over the next decade with a wide implementation of highly effective modulator treatment. It is important for respiratory clinical teams and the wider group of specialists in the multidisciplinary team to understand how these drugs are used, the side-effects and potential for drug interactions. The article by Shteinberg and Taylor-Cousar provides a very useful summary of the current position.
Full texts of both these above articles are available and are excellent.
Katharine E Secunda, Jennifer S Guimbellot, Borko Jovanovic, Sonya L Heltshe, Scott D Sagel, Steven M Rowe, Manu Jain. Females with Cystic Fibrosis Demonstrate a Differential Response Profile to Ivacaftor Compared with Males. Am J Respir Crit Care Med 2020 Apr 15;201(8):996-998.doi: 10.1164/rccm.201909-1845LE.[Pubmed]

Katherine Secunda
In this letter, the authors report three novel findings. The first is that ivacaftor-treated females with CF had a greater reduction in PEx than males with CF, noting that the baseline rate was higher in females. Second, females had a greater reduction in sweat chloride in response to ivacaftor than males. Finally, the sweat chloride response to ivacaftor is correlated with baseline weight. These data suggest that although females and males with CF showed similar salutary responses to ivacaftor in FEV1 and BMI, there may be important differential responses based on both sex and body weight.
It should be noted that ivacaftor-mediated reductions in sweat chloride have recently been shown to correlate with attenuation of FEV1 decline, which suggests that decreases in sweat chloride may also correlate with reduced lung transplantation and mortality risk. Thus, one potential implication of our data is that optimization of CFTR modulator dosing based on maximal sweat chloride reduction may lead to reduced long-term risk for lung transplantation and mortality.
Dr Katherine E Secunda is a pulmonologist at the Northwestern University Feinberg School of Medicine Chicago, Illinois.
Kevin W Southern, Jared Murphy, Ian P Sinha, Sarah J Nevitt .Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del). Cochrane Database Syst Rev 2020 Dec 17;12:CD010966. doi: 10.1002/14651858.CD010966.pub3. [Pubmed]

Kevin Southern
Authors’ conclusions: There is insufficient evidence that corrector monotherapy has clinically important effects in pwCF with F508del/F508del. Both dual therapies (lumacaftor-ivacaftor, tezacaftor-ivacaftor) result in similar improvements in QoL and respiratory function with lower pulmonary exacerbation rates. Lumacaftor-ivacaftor was associated with an increase in early transient shortness of breath and longer-term increases in blood pressure (not observed for tezacaftor-ivacaftor). Tezacaftor-ivacaftor has a better safety profile, although data are lacking in children under 12 years. In this population, lumacaftor-ivacaftor had an important impact on respiratory function with no apparent immediate safety concerns; but this should be balanced against the blood pressure increase and shortness of breath seen in longer-term adult data when considering lumacaftor-ivacaftor. There is high-quality evidence of clinical efficacy with probably little or no difference in AEs for triple (elexacaftor-tezacaftor-ivacaftor) therapy in pwCF with one or two F508del variants aged 12 years or older. Further RCTs are required in children (under 12 years) and those with more severe respiratory function.
Prof. Kevin Southern is in the Department of Women’s and Children’s Health, University of Liverpool, Liverpool, UK.
– This is a useful up-to-date review and source of reference of these treatments with a full documentation of the various trials.
Sanja Stanojevic, Katarina Vukovojac, Jenna Sykes, Felix Ratjen, Elizabeth Tullis, Anne L Stephenson. Projecting the impact of delayed access to elexacaftor/tezacaftor/ivacaftor for people with Cystic Fibrosis. J Cyst Fibros 2020Aug 5;S1569-1993(20)308092.doi10.1016/j.jcf.2020.07.017.Online ahead of print [Pubmed]

Sanja Stanojevic
Therapies that target the underlying defect in Cystic Fibrosis (CF) will likely impact the future characteristics of the CF population and healthcare utilization. The objectives of this study were to estimate the potential impact of elexacaftor/tezacaftor/ivacaftor on morbidity and mortality, and the impact of delayed access
A microsimulation transition model was applied to Canadian CF Registry data to forecast lung disease severity, pulmonary exacerbations, deaths and transplants to 2030 under three scenarios: 1) no availability of elexacaftor/tezacaftor/ivacaftor, 2) availability in 2021 (‘early’) or 3) availability in 2025 (‘delayed’). Published Phase III data on treatment effects were used to estimate transition rates between disease severity states.
Results: Under specific assumptions regarding disease state and treatment effect applied to the Canadian CF population it is projected that by 2030, early introduction of elexacaftor/tezacaftor/ivacaftor is expected to reduce the number of individuals with severe lung disease by 60% (95% CI 55.3; 63.9), increase the number of individuals with mild lung disease by 18% (95%CI 18.2; 19.0) and reduce the number of pulmonary exacerbations by 19% (95%CI 18.9; 19.5). Earlier introduction of elexacaftor/tezacaftor/ivacaftor could reduce deaths by 15% (95% 13.2; 18.4) and improve the median age of survival by 9.2 years (7.5; 10.8) over a 10-year period. The expected benefits of therapy are cumulative, therefore delayed access to elexacaftor/tezacaftor/ivacaftor will result in preventable health care utilization and deaths.
Conclusions: Delayed access to elexacaftor/tezacaftor/ivacaftor will have a negative impact on lung health and survival in the CF population.
Dr Sanja Stanojevi is at Translational Medicine, Hospital for Sick Children, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Canada; Department of Community Health and Epidemiology, Dalhousie University, Halifax, Canada.
Dr Scott D Sagel is Professor Pediatrics and Pulmonary Medicine at the Department of Pediatrics, Children’s Hospital Colorado and University of Colorado Anschutz Medical Campus, Aurora, Colorado
Koliarne Tong, Daniel Barker, Megan France, Lucy Burr, Hugh Greville, Simone Visser, Peter Middleton, Claire Wainwright, Douglas Dorahy, Peter Wark. Lumacaftor/ivacaftor reduces exacerbations in adults homozygous for Phe508del mutation with severe lung disease. J Cyst Fibros 2020 May;19(3):415-420.doi: 10.1016/j.jcf.2019.12.006. Epub 2019 Dec 15.[Pubmed]

Peter Wark

Koliarne Tong
Background: Lumacaftor/ivacaftor (LUM/IVA) improves outcomes in cystic fibrosis (CF) patients homozygous for Phe508del with ppFEV1 > 40%. There is limited safety or efficacy data in patients with ppFEV1 < 40%. We determined whether LUM/IVA in patients with ppFEV1 < 40 would reduce the rate of pulmonary exacerbations.
Methods: This was a case control study performed on patients > 12 years, homozygous for Phe508del CFTR mutation and with ppFEV1 < 40%. Control subjects were matched for age, sex and ppFEV1, and had mutations ineligible for LUM/IVA. We assessed the rate of pulmonary exacerbations requiring intravenous antibiotics, the mean rate of change in ppFEV1 over 12 months and all adverse events.
Results: Data was collected from 7 Australian CF centres on 105 patients; 72 on LUM/IVA and 33 controls. LUM/IVA demonstrated a large reduction in exacerbations with an incident rate ratio of 0.455 (95%CI; 0.306 – 0.676), p < 0.001 after adjusting for the number of exacerbations in the previous 12 months. LUM/IVA prolonged the time to first exacerbation and reduced the rate of decline in ppFEV1 over 12 months. Adverse events were common; chest tightness or dyspnoea was experienced by 55% and resulted in cessation of treatment in 32%.
Conclusions: Treatment with LUM/IVA resulted in a substantially lower rate of pulmonary exacerbations, prolonged time to first exacerbation and slowed the rate of decline of ppFEV1 in participants with severe lung disease. Adverse reactions to LUM/IVA however were unacceptably frequent and resulted in a very high discontinuation rate.
Dr Koliarne Tong is Staff Specialist in Respiratory and Sleep at the Adult Cystic Fibrosis Centre, John Hunter Hospital, Australia.
Professor Peter Wark, the corresponding author, a senior staff specialist in Respiratory and Sleep Medicine at John Hunter Hospital, Newcastle and a conjoint Professor with the University of Newcastle
Tétard C, Mittaine M, Bui S, Beaufils F, Maumus P, Fayon M, Burgel PR, Lamireau T, Delhaes L, Mas E, Enaud R. Reduced Intestinal Inflammation with Lumacaftor/Ivacaftor in Adolescents with Cystic Fibrosis. J Pediatr Gastroenterol Nutr. 2020 Jul 30. doi: 10.1097/MPG.0000000000002864. Online ahead of print. [Pubmed]
A chronic intestinal inflammation may occur in patients with cystic fibrosis (CF), while no therapeutic management is proposed. While Lumacaftor/Ivacaftor is well-known to modulate the defective cystic fibrosis transmembrane conductance regulator (CFTR) protein in lungs, no data are available on the impact of this treatment on CF intestinal disorders. The authors therefore investigated the evolution of intestinal inflammation after initiation of Lumacaftor/Ivacaftor in CF adolescents (median of follow-up: 336 days (IQR: 278;435)). Median fecal calprotectin concentrations decreased significantly after Lumacaftor/Ivacaftor initiation (102 μg/g (IQR: 69;210)) compared to the baseline (713 μg/g (IQR:148;852), p = 0.001).
To their knowledge, this study showed for the first time that CF-related intestinal inflammation is improved by Lumacaftor/Ivacaftor treatment.
From Centre Hospitalier Universitaire de Bordeaux, CHU Bordeaux, CRCM Pédiatrique, Bordeaux, France.
Silke van Koningsbruggen-Rietschel, Katja Conrath, Rainald Fischer , Sivagurunathan Sutharsan, Axel Kempa, Wolfgang Gleiber, Carsten Schwarz, Andreas Hector, Nancy Van Osselaer, Arian Pano, Sam Corveleyn, Dieudonné Bwirire, Eva Santermans, Karine Muller, Susan Bellaire, Olivier Van de Steen. . GLPG2737 in lumacaftor/ivacaftor-treated CF subjects homozygous for the F508del mutation: A randomized phase 2A trial (PELICAN). J Cyst Fibros. 2020 Mar;19(2):292-298. doi: 10.1016/j.jcf.2019.09.006. Epub 2019 Oct 5.[Pubmed]

Silke van Koningsbruggen
Background: Triple combinations of cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators demonstrate enhanced clinical efficacy in CF patients with F508del mutation, compared with modest effects of dual combinations. GLPG2737 was developed as a novel corrector for triple combination therapy.
Methods: This multicenter, randomized, double-blind, placebo-controlled, phase 2a study evaluated GLPG2737 in F508del homozygous subjects who had been receiving lumacaftor 400mg/ivacaftor 250mg for ≥12weeks. The primary outcome was change from baseline in sweat chloride concentration. Other outcomes included assessment of pulmonary function, respiratory symptoms, safety, tolerability, and pharmacokinetics.
Results: Between November 2017 and April 2018, 22 subjects were enrolled and randomized to oral GLPG2737 (75mg; n=14) or placebo (n=8) capsules twice daily for 28days. A significant decrease from baseline in mean sweat chloride concentration occurred at day 28 for GLPG2737 versus placebo (least-squares-mean difference-19.6mmol/L [95% confidence interval (CI) -36.0, -3.2], p=.0210). The absolute improvement, as assessed by least-squares-mean difference in change from baseline, in forced expiratory volume in 1s (percent predicted) at day 28 for GLPG2737 versus placebo was 3.4% (95% CI -0.5, 7.3). Respiratory symptoms in both groups remained stable. Mild/moderate adverse events occurred in 10 (71.4%) and 8 (100%) subjects receiving GLPG2737 and placebo, respectively. Lower exposures of GLPG2737 (and active metabolite M4) were observed than would be expected if administered alone (as lumacaftor induces CYP3A4). Lumacaftor and ivacaftor exposures were as expected.
Conclusions: GLPG2737 was well tolerated and yielded significant decreases in sweat chloride concentration versus placebo in subjects homozygous for F508del receiving lumacaftor/ivacaftor, demonstrating evidence of increased CFTR activity when added to a potentiator-corrector combination.
Dr Silke van Koningsbruggen-Rietschel is a Pediatric Pulmonologist and Director of the CF Clinical Research Center at the Cystic Fibrosis Center, Children’s Hospital, University of Cologne, Faculty of Medicine and University Hospital Cologne, Germany.
Guido Veit , Dillon F Da Fonte, Radu G Avramescu, Aiswarya Premchandar, Miklos Bagdany, Haijin Xu, Dennis Bensinger, Daniel Stubba, Boris Schmidt, Elias Matouk, Gergely L Lukacs. Mutation-specific dual potentiators maximize rescue of CFTR gating mutants. J Cyst Fibros 2020 Mar;19(2):236-244.doi: 10.1016/j.jcf.2019.10.011. Epub 2019 Oct 31. [Pubmed]

Guido Veit
The potentiator ivacaftor (VX-770) has been approved for therapy of 38 cystic fibrosis (CF) mutations (∼10% of the patient population) associated with a gating defect of the CF transmembrane conductance regulator (CFTR). Despite the success of VX-770 treatment of patients carrying at least one allele of the most common gating mutation G551D-CFTR, some lung function decline and P. aeruginosa colonization persist. This study aims at identifying potentiator combinations that can considerably enhance the limited channel activity of a panel of CFTR gating mutants over monotherapy.
The functional response of 13 CFTR mutants to single potentiators or systematic potentiator combinations was determined in the human bronchial epithelial cell line CFBE41o- and a subset of them was confirmed in primary human nasal epithelia (HNE).
Results: In six out of thirteen CFTR missense mutants the fractional plasma membrane (PM) activity, a surrogate measure of CFTR channel gating, reached only ∼10-50% of WT channel activity upon VX-770 treatment, indicating incomplete gating correction. Combinatorial potentiator profiling and cluster analysis of mutant responses to 24 diverse investigational potentiators identified several compound pairs that improved the gating activity of R352Q-, S549R-, S549N-, G551D-, and G1244E-CFTR to ∼70-120% of the WT. Similarly, the potentiator combinations were able to confer WT-like function to G551D-CFTR in patient-derived human nasal epithelia.
Conclusion: This study suggests that half of CF patients with missense mutations approved for VX-770 administration, could benefit from the development of dual potentiator therapy.
Wang Y, Zhao J, Cai Y, Ballard HJ. Cystic Fibrosis Transmembrane Conductance Regulator-dependent bicarbonate entry controls rat cardiomyocyte ATP release via pannexin1 through mitochondrial signalling and caspase activation. Acta Physiol (Oxf). 2020 May 9. doi: 10.1111/apha.13495. Epub ahead of print. [Pubmed]
Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in the heart, but its function there is unclear. CFTR regulates an ATP release pore in many tissues, but the identity and regulatory mechanism of the pore are unknown. The authors investigated the role of CFTR in ATP release from primary cardiomyocytes and ventricular wall in vivo.
They found that during simulated ischaemia, CFTR-dependent bicarbonate entry stimulated ATP and cytochrome c release from mitochondria; in the cytoplasm, cytochrome c activated caspase 3, which in turn activated Panx1, and ATP was released through the opened Panx1 channel.
Dr Y Wang is at the School of Biomedical Sciences, The University of Hong Kong, Pokfulam, Hong Kong.
Kael Wherry, Ian Williamson, Richard H Chapman, Karen M Kuntz Cost-Effectiveness of Ivacaftor Therapy for Treatment of Cystic Fibrosis Patients With the G551D Gating Mutation. Value Health 2020 Oct;23(10):1332-1339. doi: 10.1016/j.jval.2020.05.016. Epub 2020 Aug 16. [Pubmed]
Objectives: Cystic fibrosis (CF) is a rare genetic disease with no cure. Until recently, treatment has targeted symptoms of the disease and not the disease-causing genetic defect. Ivacaftor is included in a new class of breakthrough drugs targeting the genetic defects of CF. We sought to estimate the long-term cost-effectiveness of ivacaftor from a US payer perspective.
Methods: We developed an individual-level microsimulation model that followed a cohort of heterogeneous US CF patients over a lifetime. The primary outcome of interest was quality-adjusted life years (QALYs). We also compared unadjusted life years, count of acute pulmonary exacerbations, and count of lung transplants over a lifetime between patients treated with ivacaftor plus best supportive care and patients treated with best supportive care alone. We conducted one-way and probabilistic sensitivity analyses to test the impact of various model inputs and uncertainties.
Results: We found a substantial increase in QALYs, life years, and treatment costs over a lifetime for patients treated with ivacaftor plus best supportive care versus best supportive care alone. Discounted results for ivacaftor were 22.92 QALYs and $8 797 840 in total lifetime costs compared to 16.12 QALYs and $2 336 366 lifetime costs for best supportive care alone. The incremental cost-effectiveness ratios (ICERs) were $950 217 per QALY. Results from the probabilistic sensitivity analysis indicated a 0% chance that ivacaftor was cost-effective at a willingness-to-pay (WTP) threshold of $500 000 per QALY.
Nataliya Volkova, Kristin Moy, Jennifer Evans, Daniel Campbell , Simon Tian , Christopher Simard , Mark Higgins , Michael W Konstan, Gregory S Sawicki, Alexander Elbert, Susan C Charman, Bruce C Marshall, Diana Bilton Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J Cyst Fibros 2020 Jan;19(1):68-79.doi: 10.1016/j.jcf.2019.05.015.Epub 2019 Jun 10 [Pubmed] Free article

Nataliya Volkova
Background: Ivacaftor is the first in a class of drugs, CFTR modulators, that target the underlying defect in cystic fibrosis (CF). This long-term observational safety study evaluated CF disease progression in patients treated with ivacaftor in a real-world setting for up to 5 years.
Methods: Data from existing US and UK CF patient registries were used to assess longitudinal patterns in lung function, nutritional status, pulmonary exacerbations and hospitalizations, CF-related diabetes (CFRD), and Pseudomonas aeruginosa in ivacaftor-treated vs untreated comparator cohorts matched by age, sex, and disease severity.
Results: US analyses included 635 ivacaftor-treated patients and 1874 comparators followed for 5 years from year 1 of market availability (2012-2016). Evaluation of outcome patterns from pretreatment baseline (2011) through year 5 (2016), showed that relative to comparators, ivacaftor-treated patients had better preserved lung function (mean change in percent predicted FEV1, -0.7 percentage points with ivacaftor vs -8.3 percentage points in comparators) and improved nutritional status (mean body mass index change +2.4 kg/m2 with ivacaftor vs +1.6 kg/m2 in comparators). US patients treated with ivacaftor had significantly lower frequencies of exacerbations and hospitalizations in each of the 5 years of follow-up relative to pretreatment baseline and comparators. Favorable trends in CFRD and P. aeruginosa prevalence were also observed. Findings from the smaller UK registry were directionally similar to and consistent with US findings.
Conclusions: This observational study represents the largest longitudinal analysis of patients treated with ivacaftor in a real-world setting. The findings support disease modification by CFTR modulation with ivacaftor.
Nataliya Volkova is Senior Director Clinical Epidemiology at Vertex Pharmaceuticals Incorporated, 50 Northern Avenue, Boston, MA, USA.
2021 2021 2021
Chilvers MA, Davies JC, Milla C, Tian S, Han Z, Cornell AG, Owen CA, Ratjen F. Long-term safety and efficacy of lumacaftor-ivacaftor therapy in children aged 6-11 years with cystic fibrosis homozygous for the F508del-CFTR mutation: a phase 3, open-label, extension study. Lancet Respir Med. 2021 Jan 28:S2213-2600(20)30517-8. doi: 10.1016/S2213-2600(20)30517-8. Online ahead of print. [Pubmed]
Background: The safety and efficacy of 24 weeks of lumacaftor-ivacaftor combination therapy in children aged 6-11 years with cystic fibrosis homozygous for the F508del-CFTR mutation was previously shown in two phase 3 studies. Here, we report long-term safety and efficacy data.
Methods: In this phase 3, open-label, multicentre, extension study (study 110), we examined the long-term safety, tolerability, and efficacy of lumacaftor-ivacaftor in children pooled from two phase 3 parent studies (open-label study 011B and randomised, placebo-controlled study 109). The study was conducted at 61 clinics in the USA, Australia, Belgium, Canada, Denmark, France, Germany, Sweden, and the UK. Children with cystic fibrosis homozygous for the F508del-CFTR mutation who had received lumacaftor-ivacaftor or placebo in the parent studies were treated with lumacaftor-ivacaftor for up to 96 weeks; those who had received the combination therapy in the parent studies (the treatment-to-treatment group) received up to 120 weeks of treatment in total. Participants aged 6-11 years at the start of the parent study received lumacaftor 200 mg-ivacaftor 250 mg orally once every 12 h; those aged 12 years or older received lumacaftor 400 mg-ivacaftor 250 mg orally once every 12 h. The primary endpoint was safety and tolerability in all children who had received at least one dose of the study drug. Secondary endpoints included change from baseline in lung clearance index 2·5% (LCI2·5), sweat chloride concentration, body-mass index, and Cystic Fibrosis Questionnaire-Revised respiratory domain score. This extension study is registered with ClinicalTrials.gov, NCT02544451, and has been completed.
Findings: The extension study ran from Aug 13, 2015, to Aug 17, 2018. Of 239 children who enrolled in the study and received at least one dose of lumacaftor-ivacaftor, 215 (90%) completed 96 weeks of treatment. Most children (236 [99%] of 239 children) had adverse events that were mild (49 [21%] of 239) or moderate (148 [62%] of 239) in severity, and there was a low rate of adverse events leading to treatment discontinuation. The most frequently reported adverse events were common manifestations or complications of cystic fibrosis, such as cough and pulmonary exacerbation, or were consistent with the known safety profile of lumacaftor-ivacaftor in older children and adults. No new safety concerns were identified with extended lumacaftor-ivacaftor treatment. Children in the placebo-to-treatment group had improvements in efficacy endpoints consistent with those observed in the parent studies. Improvements observed in children treated with lumacaftor-ivacaftor in the parent study were generally maintained in the extension study.
Interpretation: Lumacaftor-ivacaftor therapy in children homozygousi for F508del-CFTR who initiated treatment at age 6-11 years was generally safe and well tolerated, and efficacy was sustained for up to 120 weeks. These data support the long-term use of lumacaftor-ivacaftor to treat children aged 6 years and older who are homozygous for the F508del-CFTR mutation.
Dr Mark Chilvers is Clinical Associate Professor, Division of Respiratory Medicine, Department of Pediatrics, Faculty of Medicine, University of British Columbia
Kavita Dave , Rebecca Dobra , Sandra Scott , Clare Saunders , Jess Matthews , Nicholas J Simmonds , Jane C Davies. Entering the era of highly effective modulator therapies. Pediatr Pulmonol 2021 Feb;56 Suppl 1:S79-S89. doi: 10.1002/ppul.24968. [Pubmed]

Kavita Dave
Since the discovery of the gene responsible for cystic fibrosis (CF) in 1989, hopes have been pinned on a future with novel therapies tackling the basis of the disease rather than its symptoms. These have become a reality over the last decade with the development through to the clinic of CF transmembrane conductance regulator (CFTR) modulators. These are oral drugs which improve CFTR protein function through either increasing the time the channel pore is open (potentiators) or facilitating its trafficking through the cell to its location on the cell membrane (correctors). The first potentiator, ivacaftor, is now licensed and available clinically in many parts of the world. It is highly effective with impressive clinical impact in the lungs and gastrointestinal tract; longer-term data from patient registries show fewer exacerbations, a slower rate of lung function loss and reduced need for transplantation in patients receiving ivacaftor. However, as a single drug, it is suitable for only a small minority of patients. The commonest CFTR mutation, F508del, requires both correction and potentiation for clinical efficacy. Two dual-agent drugs (lumacaftor/ivacaftor and tezacaftor/ivacaftor) have progressed through to licensing, although their short term impact is more modest than that of ivacaftor; this is likely due to only partial correction of protein misfolding and trafficking. Most recently, triple compounds have been developed: two different corrector molecules (elexacaftor and tezacaftor) which, by addressing different regions in the misfolded F508del protein, more effectively improve trafficking. In addition to large improvements in clinical outcomes in people with two copies of F508del, the combination is sufficiently effective that it works in patients with only one copy of F508del and a second, nonmodulator responsive mutation. For the first time, we thus have a drug suitable for around 85% of people with CF. Even more gains are likely to be possible when these drugs can be used in younger children, although more sensitive outcome measures are needed for this age group. Special consideration is needed for people with very rare mutations; those with nonmodulatable mutation combinations will likely require gene or messenger RNA-based therapeutic approaches, many of which are being explored. Although this progress is hugely to be celebrated, we still have more work to do. The international collaboration between trials networks, pharma, patient organizations, registries, and people with CF is something we are all rightly proud of, but innovative trial design and implementation will be needed if we are to continue to build on this progress and further develop drugs for people with CF.
Dr Kavita Dave is the UK CF Trust Clinical Fellow at Departments of Cystic Fibrosis and Paediatric Respiratory Medicine, Royal Brompton & Harefield Foundation Trust, London, UK
Marie E Egan. Emerging technologies for cystic fibrosis transmembrane conductance regulator restoration in all people with CF. Pediatr Pulmonol 2021 Feb;56 Suppl 1:S32-S39.doi: 10.1002/ppul.24965. [Pubmed]

Marie Egan
Although effective cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy has the potential to change the lives of many patients with cystic fibrosis (CF), it is unlikely that these drugs will be a game changing therapy for all. There are about 10% of patients with CF who don’t produce a mutant protein tomodulate, potentiate, or optimize and for these patients such therapies are unlikely to be of significant benefit. There is a need to develop new therapeutic approaches that can work for this patient population and can advance CF therapies. These new therapies will be genetic-based therapies and each approach will result in functional CFTR protein inpreviously affected CF cells. In this review we will examine the potential of RNA therapies, gene transfer therapies, and gene editing therapies for the treatment of CF as well as the challenges that will need to be facedas we harness the power of these emerging therapies towards a one-time cure.
Marie Egan is Professor of Pediatrics and of Cellular and Molecular Physiology: Director, Cystic Fibrosis Center; Vice Chair for Research Department of Pediatrics, Yale School of Medicine.
Anabela S Ramalho , Eva Fürstová , Annelotte M Vonk , Marc Ferrante, Catherine Verfaillie, Lieven Dupont , Mieke Boon , Marijke Proesmans , Jeffrey M Beekman, Ifat Sarouk, Carlos Vazquez Cordero, Francois Vermeulen, Kris De Boeck , Belgian Organoid Project. Correction of CFTR function in intestinal organoids to guide treatment of cystic fibrosis. Eur Respir J 2021 Jan 5;57(1):1902426.doi: 10.1183/13993003.02426-2019. Print 2021 Jan. [Pubmed]

Anabela Ramalho
Given the vast number of cystic fibrosis transmembrane conductance regulator (CFTR) mutations, biomarkers predicting benefit from CFTR modulator therapies are needed for subjects with cystic fibrosis (CF). To study CFTR function in organoids of subjects with common and rare CFTR mutations and evaluate correlations between CFTR function and clinical data.
Intestinal organoids were grown from rectal biopsies in a cohort of 97 subjects with CF. Residual CFTR function was measured by quantifying organoid swelling induced by forskolin and response to modulators by quantifying organoid swelling induced by CFTR correctors, potentiator and their combination. Organoid data were correlated with clinical data from the literature.
Across 28 genotypes, residual CFTR function correlated (r2=0.87) with sweat chloride values. When studying the same genotypes, CFTR function rescue by CFTR modulators in organoids correlated tightly with mean improvement in lung function (r2=0.90) and sweat chloride (r2=0.95) reported in clinical trials. We identified candidate genotypes for modulator therapy, such as E92K, Q237E, R334W and L159S. Based on organoid results, two subjects started modulator treatment: one homozygous for complex allele Q359K_T360K, and the second with mutation E60K. Both subjects had major clinical benefit.
Measurements of residual CFTR function and rescue of function by CFTR modulators in intestinal organoids correlate closely with clinical data. Our results for reference genotypes concur with previous results. CFTR function measured in organoids can be used to guide precision medicine in patients with CF, positioning organoids as a potential in vitro model to bring treatment to patients carrying rare CFTR mutations.
Dr Anabela Santo Ramalho is in Dept of Development and Regeneration, Woman and Child Unit, CF Research Lab, KU Leuven, Leuven, Belgium.
Scott D Sagel , Umer Khan , Sonya L Heltshe , John P Clancy , Drucy Borowitz, Daniel Gelfond, Scott H Donaldson, Antoinette Moran, Felix Ratjen, Jill M VanDalfsen, Steven M Rowe. Clinical Effectiveness of Lumacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del-CFTR. A Clinical Trial. Ann Am Thorac Soc 2021 Jan;18(1):75-83.doi: 10.1513/AnnalsATS.202002-144OC. [Pubmed]

Scott Segal
To evaluate the effectiveness of LUM/IVA in children (6 yr or more) and adults (more than 18 yr) in a postapproval setting. A total of 193 patients initiated LUM/IVA, and 85% completed the study through 1 year. Baseline mean percent-predicted forced expiratory volume in 1 second (ppFEV1) was 85 (standard deviation, 22.4) in this cohort. No statistically significant change in ppFEV1 was observed from baseline to any of the follow-up time points, with a mean absolute change at 12 months of -0.3 (95% confidence interval [CI], -1.8 to 1.2). Body mass index improved from baseline to 12 months (mean change, 0.8 kg/m2; P < 0.001). Sweat chloride decreased from baseline to 1 month (mean change, -18.5 mmol/L; 95% CI, -20.7 to -16.3; P < 0.001), and these reductions were sustained through the study period. There were no significant changes in hospitalization rate for pulmonary exacerbations and Pseudomonas aeruginosa infection status with treatment.
Conclusions: In this real-world multicentre cohort of children and adults, LUM/IVA treatment was associated with significant improvements in growth and reductions in sweat chloride without statistically significant or clinically meaningful changes in lung function, hospitalization rates, or P. aeruginosa infection. (NCT02477319)
Jane C Davies, Claire E Wainwright , Gregory S Sawicki, Mark N Higgins , Daniel Campbell , Christopher Harris, Paul Panorchan, Eric Haseltine, Simon Tian, Margaret Rosenfeld. Ivacaftor in Infants Aged 4 to <12 Months with Cystic Fibrosis and a Gating Mutation. Results of a Two-Part Phase 3 Clinical Trial. Am J Respir Crit Care Med 2021 Mar 1;203(5):585-593.doi: 10.1164/rccm.202008-3177OC. 33023304

Jane Davies
Rationale: We previously reported that ivacaftor was safe and well tolerated in cohorts aged 12 to <24 months with cystic fibrosis and gating mutations in the ARRIVAL study; here, we report results for cohorts aged 4 to <12 months.
Objectives: To evaluate the safety, pharmacokinetics, and pharmacodynamics of ivacaftor in infants aged 4 to <12 months with one or more gating mutations.
Methods: ARRIVAL is a single-arm phase 3 study. Infants received 25 mg or 50 mg ivacaftor every 12 hours on the basis of age and weight for 4 days in part A and 24 weeks in part B.
Measurements and Main Results: Primary endpoints were safety (parts A and B) and pharmacokinetics (part A). Secondary/tertiary endpoints (part B) included pharmacokinetics and changes in sweat chloride levels, growth, and markers of pancreatic function. Twenty-five infants received ivacaftor, 12 in part A and 17 in part B (four infants participated in both parts). Pharmacokinetics was consistent with that in older groups. Most adverse events were mild or moderate. In part B, cough was the most common adverse event (n = 10 [58.8%]). Five infants (part A, n = 1 [8.3%]; part B, n = 4 [23.5%]) had serious adverse events, all of which were considered to be not or unlikely related to ivacaftor. No deaths or treatment discontinuations occurred. One infant (5.9%) experienced an alanine transaminase elevation >3 to ≤5× the upper limit of normal at Week 24. No other adverse trends in laboratory tests, vital signs, or ECG parameters were reported. Sweat chloride concentrations and measures of pancreatic obstruction improved.
Conclusions: This study of ivacaftor in the first year of life supports treating the underlying cause of cystic fibrosis in children aged ≥4 months with one or more gating mutations.
Further detail abstracted from the full text – This study of CFTR modulation in the first year of life suggests that ivacaftor can be safely administered to infants ≥4 months of age. Our findings are consistent with observations in older children and support treating the underlying cause of CF in children ≥4 months. Ivacaftor has a favourable safety profile; it was well tolerated at both doses tested, with no new safety concerns. Results of this study suggest improvements in CFTR function, no adverse effects on growth, and reductions in lipase concentrations with ivacaftor. Improvements in FE-1 and IRT concentrations (together with lipase data) support the potential of ivacaftor to delay or possibly minimize progressive exocrine pancreatic dysfunction. Studies evaluating a larger number of children for longer periods of time are needed to further test this hypothesis. Studies of pharmacokinetics and safety in younger infants are planned.
Substantial decreases in sweat chloride concentration were seen, which is a measure of CFTR function. Although the sample sizes were small, the mean improvement in sweat chloride concentration of −55.7 (SD, 16.2) mmol/L in these infants aged 4 to <12 months appears comparable with what has been reported in older children
Improvements in concentrations of FE-1, a biomarker of exocrine pancreatic function, were seen from baseline to 24 weeks (mean [SD] increases of 164.7 [151.9] μg/g and 99.8 [138.4] μg/g in ARRIVAL and KIWI, respectively). Similarly, in the current report, mean (SD) improvement in FE-1 concentrations at Week 24 was 166.0 (140.6) μg/g. Eleven infants had baseline FE-1 concentrations of ≤200 μg/g, indicating pancreatic insufficiency, among whom seven of nine with paired data had regained pancreatic sufficiency at Week 24.
Although the clinical relevance of improvements in biomarkers of pancreatic function is unknown, the combined findings in children aged 4 to <24 months in ARRIVAL and aged 2–5 years in KIWI suggest a possible positive and protective effect of ivacaftor on pancreatic exocrine function early in life.
Jane Davies is a Professor in Paediatric Respirology & Experimental Medicine at the National Heart and Lung Institute and an Honorary Consultant in Paediatric Respiratory Medicine at the Royal Brompton & Harefield NHS Foundation Trust.
Kate M O’Shea , Orla M O’Carroll, Catherine Carroll, Brenda Grogan, Anna Connolly, Lynda O’Shaughnessy, Trevor T Nicholson, Charles G Gallagher, Edward F McKone. Efficacy of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease. Eur Respir J. 2021 Feb 25;57(2):2003079.doi: 10.1183/13993003.03079-2020. Print 2021 Feb. [Pubmed]

Kate O’Shea
No abstract but the following abstract form the article – This study shows that ELX/TEZ/IVA improves multiple outcome measures in a small cohort of 14 patients with advanced CF lung disease attending a single centre and that these improvements are similar to those seen in patients with milder disease.
Follow-up measurement dates varied with mean repeat FEV1 at 26.4±4.2 days, mean BMI at 62±35 days and mean SwCl at 64±84 days after ELX/TEZ/IVA initiation. After treatment with ELX/TEX/IVA, FEV1 improved (27.3±7.3% pred versus 36.3±16.5% pred; p<0.0001). BMI also improved (20.7±3.6 versus 22.1±3.4 kg·m−2; p<0.0001). Sweat chloride results were only available for 11 patients, mainly due to insufficient sweat volume despite multiple attempts, but also revealed significant improvement (104.9±15.04 versus 53.6±23.3 mmol·L−1; p<0.0001). Infective exacerbations requiring hospitalisation reduced in frequency (0.28±0.17 exacerbations per month in 12 months prior versus 0.04±0.07 exacerbations per month during follow-up period of 4.9 months; p<0.001)
Most significant were the reduction in requirement for intravenous antibiotic therapy as well as improvements in lung function and sweat chloride. These results were seen in both patients exposed to previous modulator therapies and in those who were CFTR modulator-naïve. There were few significant adverse events. This therapy is expected to improve the disease trajectory for many CF patients with at least one Phe508del mutation and this expectation should also apply to those groups with more advanced disease.
Kate M O’Shea is studying at the School of Medicine University College Dublin
Dr Orla M O’Carroll is a Respiratory Specialist Registrar in the Department of Respiratory medicine St Vincent’s University Hospital, Dublin
Bardin E, Pastor A, Semeraro M, Golec A, Hayes K, Chevalier B, Berhal F, Prestat G, Hinzpeter A, Gravier-Pelletier C, Pranke I, Sermet-Gaudelus I. Modulators of CFTR. Updates on clinical development and future. Eur J Med Chem. 2021 Mar 5;213:113195.doi: 10.1016/j.ejmech.2021.113195. Epub 2021 Jan 16. [Pubmed]

Emmanuelle Bardin
Cystic fibrosis (CF) is the most frequent life-limiting autosomal recessive disorder in the Caucasian population. It is due to mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. Current symptomatic CF therapies, which treat the downstream consequences of CFTR mutations, have increased survival. Better knowledge of the CFTR protein has enabled pharmacologic therapy aiming to restore mutated CFTR expression and function. These CFTR “modulators” have revolutionised the CF therapeutic landscape, with the potential to transform prognosis for a considerable number of patients. This review provides a brief summary of their mechanism of action and presents a thorough review of the results obtained from clinical trials of CFTR modulators.
Dr Emmanuelle Bardin is a post-doctoral researcher at the Institut Necker Enfants Malades. INSERM U1151, Paris, France. in 2020 she was awarded a European Cystic Fibrosis Society and Cystic Fibrosis Europe Post-Doctoral Research Fellowship to research into “Novel insights into the impact of CFTR modulators on the response of the cystic fibrosis respiratory epithelium to S. aureus infection”.
Afsoon Sepahzad, Deborah J Morris-Rosendahl, JaneDavies. Cystic Fibrosis Lung Disease Modifiers and Their Relevance in the New Era of Precision Medicine. Genes (Basel) 2021 Apr 13;12(4):562.doi: 10.3390/genes12040562. Free PMC article[Pubmed]
Our understanding of cystic fibrosis (CF) has grown exponentially since the discovery of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in 1989. With evolving genetic and genomic tools, we have come to better understand the role of CFTR genotypes in the pathophysiology of the disease. This, in turn, has paved the way for the development of modulator therapies targeted at mutations in the CFTR, which are arguably one of the greatest advances in the treatment of CF. These modulator therapies, however, do not target all the mutations in CFTRthat are seen in patients with CF and, furthermore, a variation in response is seen in patients with the same genotype who are taking modulator therapies. There is growing evidence to support the role of non-CFTR modifiers, both genetic and environmental, in determining the variation seen in CF morbidity and mortality and also in the response to existing therapies.
This review focusses on key findings from studies using candidate gene and genome-wide approaches to identify CF modifier genes of lung disease in cystic fibrosis and considers the interaction between modifiers and the response to modulator therapies. As the use of modulator therapies expands and we gain data around outcomes, it will be of great interest to investigate this interaction further. Going forward, it will also be crucial to better understand the relative influence of genomic versus environmental factors. With this understanding, we can truly begin to deliver personalised care by better profiling the likely disease phenotype for each patient and their response to treatment.
Dr Afsoon Sepahzad is in the Department of Paediatric Respiratory Medicine, Royal Brompton and Harefield Hospitals, London SW3 6NP, UK.
Jamie Duckers, Beth Lesher, Teja Thorat, Eleanor Lucas, Lisa J McGarry, Keval Chandarana, Fosca De Iorio. Real-World Outcomes of Ivacaftor Treatment in People with Cystic Fibrosis: A Systematic Review. J Clin Med 2021 Apr 6;10(7):1527.doi: 10.3390/jcm10071527. free PMC article [Pubmed]

Jamie Duckers
Cystic fibrosis (CF) is a rare, progressive, multi-organ genetic disease. Ivacaftor, a small-molecule CF transmembrane conductance regulator modulator, was the first medication to treat the underlying cause of CF. Since its approval, real-world clinical experience on the use of ivacaftor has been documented in large registries and smaller studies. Here, we systematically review data from real-world observational studies of ivacaftor treatment in people with CF (pwCF). Searches of MEDLINE and Embase identified 368 publications reporting real-world studies that enrolled six or more pwCF treated with ivacaftor published between January 2012 and September 2019. Overall, 75 publications providing data from 57 unique studies met inclusion criteria and were reviewed. Studies reporting within-group change for pwCF treated with ivacaftor consistently showed improvements in lung function, nutritional parameters, and patient-reported respiratory and sino-nasal symptoms. Benefits were evident as early as 1 month following ivacaftor initiation and were sustained over long-term follow-up. Decreases in pulmonary exacerbations, Pseudomonas aeruginosa prevalence, and healthcare resource utilization also were reported for up to 66 months following ivacaftor initiation. In studies comparing ivacaftor treatment to modulator untreated comparator groups, clinical benefits similarly were reported as were decreases in mortality, organ-transplantation, and CF-related complications. The safety profile of ivacaftor observed in these real-world studies was consistent with the well-established safety profile based on clinical trial data.
Our systematic review of real-world studies shows ivacaftor treatment in pwCF results in highly consistent and sustained clinical benefit in both pulmonary and non-pulmonary outcomes across various geographies, study designs, patient characteristics, and follow-up durations, confirming and expanding upon evidence from clinical trials.
Dr Jamie Duckers is a consultant in the All Wales Adult Cystic Fibrosis Centre University Hospital Llandough Cardiff
Kelsey Leonhardt, Elizabeth B Autry, Robert J Kuhn, Mark A Wurth. CFTR modulator drug desensitization: preserving the hope of long term improvement. Pediatr Pulmonol. 2021 Apr 29.doi: 10.1002/ppul.25437. Online ahead of print. [Pubmed]

Kelsey Leonhardt
The development of modulator therapy has, for the first time, allowed direct targeting of the underlying cause of cystic fibrosis (CF), the cystic fibrosis transmembrane conductance regulator (CFTR). Patients treated with CFTR modulators have improvement in lung function and decreased rates of pulmonary exacerbations. In 2019, elexacaftor/tezacaftor/ivacaftor was approved for use in the United States, opening these therapies to 90% of patients with CF.
Intolerable adverse drug reactions (ADRs) to CFTR modulators results in discontinuation of therapy, which can be devastating to our patients. We describe our approach to two cases, not previously reported, of rash to elexacaftor/tezacaftor/ivacaftor in patients with a previous history of cutaneous adverse reactions to dual modulator therapy that had been addressed by desensitization. Case 1 was able to tolerate elexacaftor/tezacaftor/ivacaftor after desensitization to the triple combination therapy, while in case 2 tolerance was obtained by treating through the reaction. The loss of tolerance in these patients was unexpected, and may be a common finding in patients with history of cutaneous adverse reactions to these drugs. We hope reporting our experience, including our desensitization protocol, may benefit CF patients for whom these drug reactions may be limiting access to powerful disease altering therapies
Teja Thorat, Lisa J McGarry, Krutika Jariwala-Parikh, Brendan Limone, Machaon Bonafede, Keval Chandarana, Michael W Konstan. Long-Term Impact of Ivacaftor on Healthcare Resource Utilization Among People with Cystic Fibrosis in the United States. Pulm Ther 2021 Apr 28.doi: 10.1007/s41030-021-00154-9. Online ahead of print.[Pubmed]

Teja Thorat
Introduction: Ivacaftor was first approved in 2012 for the treatment of a select population of individuals with cystic fibrosis (CF), a rare, life-shortening genetic disease. Reductions in healthcare resource utilization (HCRU) associated with ivacaftor have been observed during limited follow-up and for selected outcomes in real-world studies. This study aimed to further describe the long-term impact of ivacaftor treatment on multiple measures of HCRU among people with CF (pwCF).
Methods: This retrospective study used US commercial and Medicaid claims data from 2011-2018. We included pwCF ≥ 6 years of age with ≥ 1 claim for ivacaftor and 12 months of continuous health plan enrollment before ivacaftor initiation (“pre-ivacaftor” period) who also had 36 months of continuous enrolment and persistent ivacaftor use (i.e., no gap ≥ 90 days between refills) following initiation (“post-ivacaftor” period). We compared comorbidities occurring pre-ivacaftor versus the last 12 months post-ivacaftor. HCRU outcomes included medication use, inpatient admissions, and outpatient office visits. We compared medication use pre-ivacaftor versus the last 12 months post-ivacaftor and inpatient admissions and outpatient office visits pre-ivacaftor versus the post-ivacaftor period annualized across 36 months.
Results: Seventy-nine pwCF met all criteria, including persistent ivacaftor use during the post-ivacaftor period. Ivacaftor treatment was associated with a significant reduction in pneumonia prevalence (10.1% vs. 26.6%; p < 0.001) and significantly fewer mean [SD] antibiotics claims (8.0 [7.3] vs. 12.3 [11.1]; p < 0.001) in the last 12 months post-ivacaftor versus pre-ivacaftor. In comparing the 36-month post-ivacaftor period to the pre-ivacaftor period, we also observed fewer mean [SD] annual inpatient admissions (0.2 [0.4] vs. 0.4 [0.7]), CF-related inpatient admissions (0.1 [0.2] vs. 0.2 [0.5]), and outpatient office visits (8.8 [4.9] vs. 9.9 [5.4]) (all, p < 0.05).
Conclusion: Long-term ivacaftor treatment reduced the health care resource utilisation (HCRU), consistent with trends observed in prior real-world studies. Our results support the sustained, long-term value of ivacaftor treatment in reducing CF burden.
Teja Thorat is a scientist who is Associate Director Health Economics and Outcomes Research at Vertex Pharmaceuticals Incorporated, Boston, MA. USA
Dr Kelsey Leonhardt in Clinical Pharmacist in the Department of Pharmacy Services, University of Kentucky HealthCare, Lexington, Kentucky.
Anne H Neerincx, Katrine Whiteson, Joann L Phan, Paul Brinkman, Mahmoud I Abdel-Aziz, Els J M Weersink, Josje Altenburg, Christof J Majoor, Anke H Maitland-van der Zee, Lieuwe D J Bos. Lumacaftor/ivacaftor changes the lung microbiome and metabolome in cystic fibrosis patients. ERJ Open Res 2021 Apr 19;7(2):00731-2020.doi: 10.1183/23120541.00731-2020.eCollection 2021 Apr. Free PMC article [Pubmed] (please see PubMed abstract for more detail)

Anne Neerincx
Targeted cystic fibrosis (CF) therapy with lumacaftor/ivacaftor partly restores chloride channel function and improves epithelial fluid transport in the airways. Consequently, changes may occur in the microbiome, which is adapted to CF lungs
A study to investigate the effects of lumacaftor/ivacaftor on respiratory microbial composition and microbial metabolic activity by repeatedly sampling the lower respiratory tract.
Findings – After starting CF transmembrane conductance regulator (CFTR) modulating treatment in CF patients with a homozygous Phe508del mutation, a temporary and moderate change in the lung microbiome is observed, which is mainly characterised by a reduction in the relative abundance of Pseudomonas aeruginosa.
Dr Anne H Neerincx is a Postdoctoral Researcher in the Dept of Respiratory Medicine, Amsterdam UMC – Location AMC, University of Amsterdam, Amsterdam, the Netherlands.
Jordana E Hoppe, Mark Chilvers, Felix Ratjen, John J McNamara, Caroline A Owen, Simon Tian, Rachel Zahigian, Alexandra G Cornell, Susanna A McColley. Long-term safety of lumacaftor-ivacaftor in children aged 2-5 years with cystic fibrosis homozygous for the F508del-CFTR mutation: a multicentre, phase 3, open-label, extension study. Lancet Respir Med 2021 May 6;S2213-2600(21)00069-2.doi: 10.1016/S2213-2600(21)00069-2. Online ahead of print. [Pubmed]

Jordana Hoppe
[Background: A previous phase 3 study showed that lumacaftor-ivacaftor was generally safe and well tolerated over 24 weeks of treatment in children aged 2-5 years with cystic fibrosis homozygous for the F508del-CFTR mutation. In this study, we aimed to assess the long-term safety of lumacaftor-ivacaftor in a rollover study of children who participated in this previous phase 3 study.
Methods: In this multicentre, phase 3, open-label, extension study (study 116; VX16-809-116), we assessed safety of lumacaftor-ivacaftor in children included in a previous multicentre, phase 3, open-label study (study 115; VX15-809-115). The study was done at 20 cystic fibrosis care centres in the USA and Canada. Children aged 2-5 years with cystic fibrosis homozygous for the F508del-CFTR mutation who completed 24 weeks of lumacaftor-ivacaftor treatment in study 115 received weight-based and age-based doses of oral lumacaftor-ivacaftor: children weighing less than 14 kg and aged younger than 6 years at study 116 screening received lumacaftor 100 mg-ivacaftor 125 mg every 12 h; children weighing 14 kg or more and aged younger than 6 years at screening received lumacaftor 150 mg-ivacaftor 188 mg every 12 h; and children aged 6 years or older received lumacaftor 200 mg-ivacaftor 250 mg every 12 h. Children received treatment for up to 96 weeks, equivalent to up to 120 weeks of treatment in total from the start of study 115 to completion of study 116. The primary endpoint was the safety and tolerability of the study drug in all participants who had received lumacaftor-ivacaftor for 24 weeks in study 115 and had received at least one dose in study 116. Secondary endpoints included change from baseline in study 115 at week 96 of study 116 in sweat chloride concentration, growth parameters, markers of pancreatic function, and lung clearance index (LCI) parameters in all children who received at least one dose of lumacaftor-ivacaftor in study 116. This study is registered with ClinicalTrials.gov, NCT03125395.
Findings: This extension study ran from May 12, 2017, to July 17, 2019. Of 60 participants enrolled and who received lumacaftor-ivacaftor in study 115, 57 (95%) were included in study 116 and continued to receive the study drug. A total of 47 (82%) of 57 participants completed 96 weeks of treatment. Most participants (56 [98%] of 57) had at least one adverse event during study 116, most of which were mild (19 [33%] participants) or moderate (29 [51%] participants) in severity. The most common adverse events were cough (47 [82%] participants), nasal congestion (25 [44%] participants), pyrexia (23 [40%] participants), rhinorrhoea (18 [32%] participants), and vomiting (17 [30%] participants). A total of 15 (26%) participants had at least one serious adverse event; most were consistent with underlying cystic fibrosis or common childhood illnesses. Respiratory adverse events occurred in five (9%) participants, none of which were serious or led to treatment discontinuation. Elevated aminotransferase concentrations, most of which were mild or moderate in severity, occurred in ten (18%) participants. Three (5%) participants discontinued treatment due to adverse events (two due to increased aminotransferase concentrations [one of whom had concurrent pancreatitis], considered as possibly related to study drug; and one due to gastritis and metabolic acidosis, considered unlikely to be related to study drug). No clinically significant abnormalities or changes were seen in electrocardiograms, vital signs, pulse oximetry, ophthalmological examinations, or spirometry assessments. Improvements in secondary endpoints observed in study 115 were generally maintained up to week 96 of study 116, including improvements in sweat chloride concentration (mean absolute change from study 115 baseline at week 96 of study 116 -29·6 mmol/L [95% CI -33·7 to -25·5]), an increase in growth parameters and pancreatic function, and stable lung function relative to baseline, as measured by the LCI.
Interpretation: Lumacaftor-ivacaftor was generally safe and well tolerated, and treatment effects were generally maintained for the duration of the extension study. These findings support the use of lumacaftor-ivacaftor for up to 120 weeks in young children with cystic fibrosis aged 2 years and older homozygous for the F508del-CFTR mutatio
Dr Jordana Hoppe, MD, a pediatric pulmonologist with Children’s Hospital Colorado and assistant professor of pediatrics at the University of Colorado School of Medicine on the Anschutz Medical Campus
Zumi Mehta, Khalid M Kamal, Richard Miller, Jordan R Covvey, Vincent Giannetti. Adherence to cystic fibrosis transmembrane conductance regulator (CFTR) modulators: analysis of a national specialty pharmacy database, J Drug Assess. 2021 Apr 5;10(1):62-67.doi: 10.1080/21556660.2021.1912352. Free PMC article [Pubmed]

Zumi Mehta
Objective: To calculate the medication adherence in patients taking CFTR modulators using a national specialty pharmacy database.
Methods: This retrospective observational cohort study utilized de-identified specialty pharmacy data from September 2017 to August 2018 to assess medication adherence for three CFTR modulators: ivacaftor, lumacaftor/ivacaftor, and tezacaftor/ivacaftor & ivacaftor. The primary outcome was proportion of days covered (PDC) for each medication, with mean PDC values compared across age groups and insurance characteristics. All analyses were performed using the SAS 9.4 University Edition (SAS Institute, Cary, NC).
Results: A total of 2,548 patients were analyzed, including 1,289 (50.59%) patients on lumacaftor/ivacaftor, 784 (30.77%) on ivacaftor, and 475 (18.64%) on tezacaftor/ivacaftor & ivacaftor. The mean PDC value for all CFTR modulators was above 0.80. Tezacaftor/ivacaftor & ivacaftor had the highest overall PDC of 0.92, while PDC values for both lumacaftor/ivacaftor and ivacaftor were 0.84. Children/adolescents on lumacaftor/ivacaftor (p = 0.0001) and tezacaftor/ivacaftor & ivacaftor (p = 0.001) had significantly higher mean PDC values compared to adults but not for ivacaftor (p = 0.3744). No statistical differences were seen in PDC across insurance characteristics.
Conclusion: To the best of our knowledge, this is the first study to assess the adherence of three CFTR modulators using a large nationwide specialty database. With high acquisition costs of CFTR modulator therapies, there is a need to improve rates of adherence in patients with CF
Dr Zumi Mehta is graduate student in the Graduate School of Pharmaceutical Sciences, Duquesne University, Pittsburgh, PA, USA.
Justin D Anderson, Zhongyu Liu, L Victoria Odom, Latona Kersh, Jennifer S Guimbellot. CFTR Function and Clinical Response to Modulators Parallel Nasal Epithelial Organoid Swelling. Am J Physiol Lung Cell Mol Physiol 2021 May 19.doi: 10.1152/ajplung.00639.2020.Online ahead of print. [Pubmed]
Rationale: In vitro biomarkers to assess Cystic Fibrosis Transmembrane Conductance Regulator activity are desirable for precision modulator selection and as a tool for clinical trials.
Objectives: We describe an organoid swelling assay derived from human nasal epithelia using commercially available reagents and equipment and an automated imaging process.
Methods: Cells were collected in nasal brush biopsies, expanded in vitro, and cultured as spherical organoids or as monolayers. Organoids were used in a functional swelling assay with automated measurements and analysis, while monolayers were used for short-circuit current measurements to assess ion channel activity. Clinical data was collected from patients on modulators. Relationships between swelling data and short-circuit current, as well as between swelling data and clinical outcome measures, were assessed.
Main results: The organoid assay measurements correlate with short-circuit current measurements for ion channel activity. The functional organoid assay distinguished individual responses as well as differences between groups. The organoid assay distinguished incremental drug responses to modulator monotherapy with ivacaftor and combination therapy with ivacaftor, tezacaftor, and elexacaftor. The swelling activity paralleled clinical response.
Conclusions: An in vitro biomarker derived from patients’ cells can be used to predict responses to drugs and is likely to be useful as a pre-clinical tool to aid in development of novel treatments, and as a clinical trial outcome measure for a variety of applications, including gene therapy or editing.
Dr Justin D Anderson is at the Gregory Fleming James Cystic Fibrosis Research Center, grid.265892.2University of Alabama at Birmingham, Birmingham, AL, and the Department of Pediatrics, Division of Pulmonary and Sleep Medicine, grid.265892.2University of Alabama at Birmingham, Birmingham, AL, USA.
Duncan E Keegan, John J Brewington. Nasal Epithelial Cell-Based Models for Individualized Study in Cystic Fibrosis. Int J Mol Sci 2021 Apr 24;22(9):4448.doi: 10.3390/ijms22094448. Free article [Pubmed]
The emergence of highly effective CFTR modulator therapy has led to significant improvements in health care for most patients with cystic fibrosis (CF). For some, however, these therapies remain inaccessible due to the rarity of their individual CFTR variants, or due to a lack of biologic activity of the available therapies for certain variants. One proposed method of addressing this gap is the use of primary human cell-based models, which allow preclinical therapeutic testing and physiologic assessment of relevant tissue at the individual level. Nasal cells represent one such tissue source and have emerged as a powerful model for individual disease study. The ex vivo culture of nasal cells has evolved over time, and modern nasal cell models are beginning to be utilized to predict patient outcomes. This review will discuss both historical and current state-of-the art use of nasal cells for study in CF, with a particular focus on the use of such models to inform personalized patient care.
Dr Duncan E Keegan is a Pulmonary Fellow in the Division of Pulmonary Medicine, Cincinnati Children’s Hospital Medical Center USA.and the Department of Pediatrics, University of Cincinnati College of Medicine.
Dr John Brewington is a pediatric pulmonologist at Cincinnati-Children’s-Hospital-Medical-Center with an interest in CF and other airway disorders.
Renske van der Meer, Erik B Wilms, Harry G M Heijerman. CFTR Modulators: Does One Dose Fit All? J Pers Med 2021 May 24;11(6):458.doi: 10.3390/jpm11060458. [Pubmed]

Renske van der Meer
For many people with cystic fibrosis (pwCF), CFTR modulators will be the cornerstone of their treatment. These modulators show robust treatment effects at group level in pwCF with specific mutations. The individual effect however, is variable. In this review we will explain reasons for reconsideration of dosing regimens of CFTR modulating therapy in order to improve treatment response and prevent side effects. Since the effect of a drug depends on pharmacodynamics and pharmacokinetics, pharmacodynamics and pharmacokinetic properties of CFTR modulators will be discussed. Pharmacokinetic-pharmacodynamic relationships will be used to gain insight in dosage response and exposure response relationships. To understand the cause of variation in drug exposure, pharmacokinetic properties that may change due to CF disease will be explained. We show that with current insight, there are conceivable situations that give reason for reconsideration of dosing regimens, however many questions need to be unravelled.
Dr Renske van der Meer is in the Department of Pulmonology, Haga Teaching Hospital, Els Borst-Eilersplein 275, 2545 AA The Hague, The Netherlands
(The Journal of Personalized Medicine is an open access journal)
Dr Morgan Green is in the Department of Medicine, University of Alabama at Birmingham, United States
Simon Y Graeber, Constanze Vitzthum, Marcus A Mall. Potential of Intestinal Current Measurement for Personalized Treatment of Patients with Cystic Fibrosis. J Pers Med. 2021 May 8;11(5):384.doi: 10.3390/jpm11050384.[Pubmed]Free PMC article

Simon Graeber
Refinement of personalized treatment of cystic fibrosis (CF) with emerging medicines targeting the CF basic defect will likely benefit from biomarkers sensitive to detect improvement of cystic fibrosis transmembrane conductance regulator (CFTR) function in individual patients. Intestinal current measurement (ICM) is a technique that enables quantitative assessment of CFTR chloride channel function in rectal tissues or other intestinal epithelia. ICM was originally developed to study the CF ion transport defect in the intestine and has been established as a sensitive biomarker of CFTR function and diagnostic test for CF. With the emergence of CFTR-directed therapeutics, ICM has become an important tool to estimate the level of rescue of CFTR function achieved by approved CFTR modulators, both at the level of CFTR genotype groups, as well as individual patients with CF. In combination with preclinical patient-derived cell culture models, ICM may aid the development of targeted therapies for patients with rare CFTR mutations. Here, we review the principles of ICM and examine how this CFTR biomarker may be used to support diagnostic testing and enhance personalized medicine for individual patients with common as well as rare CFTR mutations in the new era of medicines targeting the underlying cause of CF.
Dr Simon Y Graeber is at the Charité-Universitätsmedizin Berlin, Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine, 13353 Berlin, Germany.
Iona Paterson, Chris Johnson, Gordon MacGregor. Tezacaftor-ivacaftor use in routine care of adults with cystic fibrosis: a medicine use evaluation. Eur J Hosp Pharm 2021 Jun 8;ejhpharm-2020-002676.doi: 10.1136/ejhpharm-2020-002676.Online ahead of print [Pubmed]
This study aimed to assess the impact of tezacaftor-ivacaftor use in routine clinical practice for adults with cystic fibrosis.
Methods: A retrospective observational longitudinal cohort study design was applied to examine the clinical effect of tezacaftor-ivacaftor in routine practice in the West of Scotland Adult Cystic Fibrosis Unit. Adults receiving tezacaftor-ivacaftor for at least 4 weeks were included in this medicine use evaluation. A standardised data form was used to collect patient-level data: demographics, genotype, complications of cystic fibrosis, medicine access process. Fifty-two weeks pre and post tezacaftor-ivacaftor initiation data: lung function, body mass index (BMI), days spent in hospital, days receiving antibiotic treatment for respiratory exacerbations. Anonymised data were collated and analysed using SPSS V.26.
Results: Of 121 potential patients, 45 received treatment with tezacaftor-ivacaftor; median age 30 years (range 17-64) at initiation, 56% were male, 76% were deemed to be homozygote and 41 patients continued treatment for at least 52 weeks. There was no significant change in % predicted FEV1; median difference 0 (IQR -3 to 6). There was a significant improvement in BMI, mean 0.6 kg/m2 (95% CI 0.2 to 1.0), as well as a median 4 (IQR -17 to 0) day reduction in days in hospital and 21 (IQR -42 to 0) day reduction in days receiving antibiotics.
Conclusions: The use of tezacaftor-ivacaftor in routine practice for people with cystic fibrosis was associated with improvements in weight, as well as reducing the number of days people needed to spend in hospital and receive antibiotics.
Iona Paterson is Pharmacist at the Queen Elizabeth University Hospital Campus, Glasgow, UK
Dominique Hubert, Christophe Marguet, Jacques Benichou, Cynthia DeSouza, Catherine Payen-Champenois, Nils Kinnman, Keval Chandarana, Anne Munck, Isabelle Fajac , BRIO Study Group. Real-World Long-Term Ivacaftor for Cystic Fibrosis in France: Clinical Effectiveness and Healthcare Resource Utilization. Pulm Ther 2021 Jun 8.doi: 10.1007/s41030-021-00158-5. Online ahead of print. [Pubmed]

Isabelle Fajac

Dominique Hubert
Introduction: Ivacaftor is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator that has demonstrated clinical benefits in phase 3 trials. We report results from a real-world study (BRIO) to assess the effectiveness of ivacaftor in people with cystic fibrosis (pwCF) in France.
Methods: BRIO was an observational study conducted at 35 centers in France. Both pwCF initiating ivacaftor treatment and those already taking ivacaftor were included and prospectively followed for 24 months. The primary objective was to evaluate the effect of ivacaftor on percent predicted forced expiratory volume in 1 s (ppFEV1); secondary objectives were evaluating the effect of ivacaftor on clinical effectiveness, healthcare resource utilization (HCRU), and safety.
Results: A total of 129 pwCF were enrolled; 58.9% were aged < 18 years; 64.3% had a G551D-CFTR allele. Mean age at ivacaftor initiation was 19.1 years (range, 2-64 years); ppFEV1 increased by a least squares mean of 8.49 percentage points in the first 6 months and was sustained through 36 months of ivacaftor use. Growth metrics increased during the first 12 months post-ivacaftor and remained stable. The rate of pulmonary exacerbations (PEx) decreased during the 12 months post-fivacaftor compared with the 12 months pre-ivacaftor; estimated rate ratios (95% CI) were 0.57 (0.43-0.75) for PEx events and 0.25 (0.13-0.48) for PEx requiring hospitalization. No new safety concerns were identified; no deaths occurred.
Conclusions: The results from this real-world study of ivacaftor usage in France were consistent with prior clinical trial outcomes, confirming the clinical effectiveness of ivacaftor, as well as an associated reduction in health care resource utilization
Dr Dominique Hubert is at the Respiratory Medicine and National Cystic Fibrosis Reference Center, Cochin Hospital, Assistance Publique-Hôpitaux de Paris, Paris, France.
Dr Isabelle Fajac is at the Respiratory Medicine and National Cystic Fibrosis Reference Center, Cochin Hospital, Assistance Publique-Hôpitaux de Paris; the Physiology Department, AP-HP Centre-Université de Paris, Hôpital Cochin and the Université de Paris, Paris, France.
Dr Jordana Hoppe, MD, a pediatric pulmonologist with Children’s Hospital Colorado and assistant professor of pediatrics at the University of Colorado School of Medicine on the Anschutz Medical Campus
Philippe Reix, Aurélie Tatopoulos, Iulia Ioan, Muriel Le Bourgeois, Stéphanie Bui, Marie Luce Choukroun, Katia Bessaci-Kabouya, Michele Gerardin, Plamen Bokov, Jennifer Da Silva, Jean-Louis Paillasseur, Pierre Regis Burgel, French Cystic Fibrosis Reference Network study group. Real-world assessment of LCI following lumacaftor-ivacaftor initiation in adolescents and adults with cystic fibrosis. J Cyst Fibros 2021 Jun 25;S1569-1993(21)01286-8.doi: 10.1016/j.jcf.2021.06.002.Online ahead of print. [Pubmed]
Lung clearance index (LCI) is a biomarker of ventilation inhomogeneity. Data are scarce on its usefulness in daily practice for monitoring the effects of treatments in older children and adults with CF. In this French observational study of lumacaftor-ivacaftor, 63 of 845 patients (7.5%) had available LCI performed at baseline and at six (M6; n=34) or 12 months (M12; n=46) after lumacaftor-ivacaftor initiation. At inclusion, median [IQR] age was 16 years [13-17], ppFEV1 was 72.8 [59.6-80.7], and LCI was 12.3 [10.3-15.0].
At both M6 and M12, non statistically significant LCI increases of 0.13 units or 1.34% (95% CI: -4.85-7.53) and 0.6 units or 6.66% (95% CI: -0.03-13.5) were observed. Discordant results between LCI and ppFEV1 were observed in one-third of the patients.
In daily practice, LCI monitoring in adolescents and young adults with moderate lung disease gives results that are more heterogenous than those reported in children with milder disease.
Dr Philippe Reix is at the Cystic Fibrosis Center, Hospices Civils de Lyon, Lyon, France; UMR 5558 CNRS Equipe EMET Université Claude Bernard Lyon 1 Lyon, France.
Frédéric Becq, Sandra Mirval, Thomas Carrez, Manuella Lévêque, Arnaud Billet, Christelle Coraux, Edouard Sage, Anne Cantereau. The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor. Eur Respir J 2021 Jul 15;2100671.doi: 10.1183/13993003.00671-2021.Online ahead of print. [Pubmed]

Frederic Becq
Trikafta, currently the leading therapeutic in Cystic Fibrosis (CF), has demonstrated a real clinical benefit. This treatment is the triple combination therapy of two folding correctors elexacaftor/tezacaftor (VX445/VX661) plus the gating potentiator ivacaftor (VX770). In this study, our aim was to compare the properties of F508del-CFTR in cells treated with either lumacaftor (VX809), tezacaftor, elexacaftor, elexacaftor/tezacaftor with or without ivacaftor. We studied F508del-CFTR function, maturation and membrane localisation by Ussing chamber and whole-cell patch clamp recordings, Western blot and immunolocalization experiments. With human primary airway epithelial cells and the cell lines CFBE and BHK expressing F508del, we found that, whereas the combination elexacaftor/tezacaftor/ivacaftor was efficient in rescuing F508del-CFTR abnormal maturation, apical membrane location and function, the presence of ivacaftor limits these effects. The basal F508del-CFTR short-circuit current was significantly increased by elexacaftor/tezacaftor/ivacaftor and elexacaftor/tezacaftor compared to other correctors and non-treated cells, an effect dependent on ivacaftor and cAMP. These results suggest that the level of the basal F508del-CFTR current might be a marker for correction efficacy in CF cells. When cells were treated with ivacaftor combined to any correctors, the F508del-CFTR current was unresponsive to the subsequently acute addition of ivacaftor unlike the CFTR potentiators genistein and Cact-A1 which increased elexacaftor/tezacaftor/ivacaftor and elexacaftor/tezacaftor-corrected F508del-CFTR currents. These findings show that ivacaftor reduces the correction efficacy of Trikafta. Thus, combining elexacaftor/tezacaftor with a different potentiator might improve the therapeutic efficacy for treating CF patients
Professor Frédéric Becq is at the Laboratoire Signalisation et Transports Ioniques Membranaires, Université de Poitiers, France.
Guido Veit, Christian Vaccarin, Gergely L Lukacs. Elexacaftor co-potentiates the activity of F508del and gating mutants of CFTR. J Cyst Fibros 2021 Mar 25;S1569-1993(21)00087-4.doi: 10.1016/j.jcf.2021.03.011.Online ahead of print. [Pubmed]

Guido Veit
Trikafta, the combination of elexacaftor (VX-445), tezacaftor (VX-661) and ivacaftor (VX-770), was approved for therapy of cystic fibrosis (CF) patients with at least one allele of the CFTR mutation F508del. While the corrector function of VX-445 is well established, here we investigated the putative potentiator activity of VX-445 alone and in combination with VX-770. Acute addition of VX-445 increased the VX-770-potentiated F508del- and G551D-CFTR current by ~24% and >70%, respectively, in human bronchial and nasal epithelia. Combinatorial profiling and cluster analysis of G551D- and G1244E-CFTR channel activation with potentiator pairs indicated a distinct VX-445 mechanism of action that is, at least, additive to previously identified potentiator classes, including the VX-770. Since VX-770 only partially normalizes the G551D-CFTR channel function and adult G551D patients still experience progressive loss of lung function, VX-445+VX-770 combination therapy could provide clinical benefit to CF patients with the G551D and other dual potentiator responsive mutants
Dr Guido Velt is in the Department of Physiology, McGill University, Montréal, QC H3G 1Y6, Canada.
Marika Comegna , Vito Terlizzi, Donatello Salvatore, Carmela Colangelo, Antonella Miriam Di Lullo, Immacolata Zollo , Giovanni Taccetti, Giuseppe Castaldo, Felice Amato. Elexacaftor-Tezacaftor-Ivacaftor Therapy for Cystic Fibrosis Patients with The F508del/Unknown Genotype. Antibiotics (Basel)2021 Jul 7;10(7):828.doi: 10.3390/antibiotics10070828. Free PMC article [Pubmed]

Marika Comegna
The new CFTR modulator combination, elexacaftor/tezacaftor/ivacaftor (Trikafta) was approved by the FDA in October 2019 for treatment of Cystic Fibrosis in patients 6 years of age or older who have at least one F508del mutation in one allele and a minimal-function or another F508del mutation in the other allele. However, there is a group of patients, in addition to those with rare mutations, in which despite the presence of a F508del in one allele, it was not possible to identify any mutation in the other allele. To date, these patients are excluded from treatment with Trikafta in Italy, where the CF patients carrying F508del/unknown represent about 1.3% (71 patients) of the overall Italian CF patients. In this paper we show that the Trikafta treatment of nasal epithelial cells, derived from F508del/Unknown patients, results in a significant rescue of CFTR activity. Based on our findings, we think that the F508del/Unknown patients considered in this study could obtain clinical benefits from Trikafta treatment, and we strongly suggest their eligibility for this type of treatment. This study, adding further evidence in the literature, once again confirms the validity of functional studies on nasal cells in the cystic fibrosis theratyping and personalized medicine
Dr Marika Comegna is in the Department of Molecular Medicine and Medical Biotechnologies, University of Naples Federico II, Via Pansini, 5, 80131 Naples, Italy.
CEINGE-Advanced Biotechnologies, Via G. Salvatore, 486, 80145 Naples, Italy.
Scott D Sagel, Umer Khan, Sonya L Heltshe, John P Clancy, Drucy Borowitz, Daniel Gelfond, Scott H Donaldson, Antoinette Moran, Felix Ratjen, Jill M VanDalfsen, Steven M Rowe. Clinical Effectiveness of Lumacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del-CFTR. A Clinical Trial. Ann Am Thorac Soc 2021 Jan;18(1):75-83.doi: 10.1513/AnnalsATS.202002-144OC. [Pubmed]

Scott Segal
To evaluate the effectiveness of LUM/IVA in children (6 yr or more) and adults (more than 18 yr) in a postapproval setting. A total of 193 patients initiated LUM/IVA, and 85% completed the study through 1 year. Baseline mean percent-predicted forced expiratory volume in 1 second (ppFEV1) was 85 (standard deviation, 22.4) in this cohort. No statistically significant change in ppFEV1 was observed from baseline to any of the follow-up time points, with a mean absolute change at 12 months of -0.3 (95% confidence interval [CI], -1.8 to 1.2). Body mass index improved from baseline to 12 months (mean change, 0.8 kg/m2; P < 0.001). Sweat chloride decreased from baseline to 1 month (mean change, -18.5 mmol/L; 95% CI, -20.7 to -16.3; P < 0.001), and these reductions were sustained through the study period. There were no significant changes in hospitalization rate for pulmonary exacerbations and Pseudomonas aeruginosa infection status with treatment.
Conclusions: In this real-world multicentre cohort of children and adults, LUM/IVA treatment was associated with significant improvements in growth and reductions in sweat chloride without statistically significant or clinically meaningful changes in lung function, hospitalization rates, or P. aeruginosa infection. (NCT02477319).
Dr Scott D Sagel is Professor Pediatrics and Pulmonary Medicine at the Department of Pediatrics, Children’s Hospital Colorado and University of Colorado Anschutz Medical Campus, Aurora, Colorado.
Eva Furstova, Tereza Dousova, Jakub Beranek, Malgorzata Libik, Libor Fila, Martin Modrak, Ondrej Cinek, Milan Macek Jr, Pavel Drevinek Response to elexacaftor/tezacaftor/ivacaftor in intestinal organoids derived from people with cystic fibrosis. J Cyst Fibros 2021 Aug 1;S1569-1993(21)01304-7.doi: 10.1016/j.jcf.2021.07.006.Online ahead of print.[Pubmed]

Eva Furstova
Superior efficacy of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) over tezacaftor/ivacaftor (TEZ/IVA) in people with cystic fibrosis (CF) and Phe508del/Phe508del genotype was shown in clinical trials. We utilized intestinal organoid approach to compare in vitro responses to these 2 CFTR modulator drug combinations and to check potential inter-individual variability in therapeutic response to the triple combination.
Organoids from 17 subjects with Phe508del/Phe508del were screened with forskolin induced swelling assay. Significantly larger swelling, when exposed to ELX/TEZ/IVA as compared to TEZ/IVA, was observed in 16 of them. However, 1 sample showed no additional effect of ELX. The finding of unique CFTR variants in this sample indicates that genetic traits other than CF-causing CFTR mutation are worth exploring as they may have an impact on the definitive modulator drug response.
Dr Eva Furstova is a medical doctor and PhD researcher in the Department of Paediatrics, 2nd Faculty of Meicine, Charles University and Motol University Hospital, Prague, Czech Republic; Department of Medical Microbiology, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic
James M Clegg, Kelsey W Malloy, Rebekah F Brown, Alison G Grisso, Andrew G Sokolow. Ivacaftor withdrawal syndrome: A potentially life-threatening consequence from a life-saving medication. J Cyst Fibros. 2021 Aug 11;S1569-1993(21)01340-0. doi: 10.1016/j.jcf.2021.07.021.Online ahead of print. Pubmed

James Clegg
In 2018, a case series of three adult patients with cystic fibrosis (CF) suggested an ivacaftor withdrawal syndrome (IWS) perpetuated by abrupt cessation of ivacaftor treatment . An additional case published that year proposed IWS in the setting of rifampin use, demonstrating this may also be induced by drug-drug interactions . While this syndrome is a rare complication of highly effective modulator therapy (HEMT), it is important to recognize as increasing numbers of patients are prescribed HEMT. Adult patients are not solely affected, as demonstrated by a pediatric patient’s recent hospitalization.
A 12-year-old patient with CF (delF508/G1244E), who had been receiving ivacaftor therapy for four months, presented to the emergency room with fever, malaise, vomiting, productive cough, pleuritic chest pain, and dyspnea for three days. Physical exam showed tachypnea, hypoxemia, decreased breath sounds and respiratory crackles. Laboratory studies revealed leukocytosis and a negative PCR of common respiratory pathogens via nasal swab. Sputum culture grew chronic methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa without a change in antibiotic resistance from previous cultures. Blood culture was negative. Chest radiograph was stable without acute changes. Spirometry on admission revealed all-time low pulmonary function for this patient with a forced expiratory volume in one second (FEV 1 ) of 27% predicted value (%pv) and forced vital capacity (FVC) of 32%pv, decreased from recent baseline values of 77%pv and 83%pv, respectively. The patient was admitted for aggressive airway clearance, intravenous antibiotics, and supplemental oxygen up to four liters per minute for hypoxemia. Secondary to her degree of distress, bronchoalveolar lavage was not possible at the time of presentation. She had required 12 prior CF-related hospitalizations, during which she presented with a decline of FEV 1 by 25%pv or less and displayed no hypoxemia or requirement for respiratory support.
Her family informed the medical team of a two-week lapse in taking her ivacaftor due to a combination of language barrier, difficulty in reaching the family by pharmacy, and miscommunication amongst her numerous caregivers regarding dosages given. IWS was suspected given severity of symptoms and lack of other likely precipitating factors. Sweat chloride testing was obtained and resulted elevated. Ivacaftor was reinitiated on hospital day two with the arrival of the patient’s home supply. One day after reinitiating ivacaftor, she defervesced, and tachypnea and hypoxemia resolved. Five days after reinitiation, repeat sweat chloride was within normal range. Eight weeks post-discharge, FEV 1 was 76%pv. She transitioned to elexacaftor/tezacaftor/ivacaftor (elex/tez/iva) therapy leading to further improvement in pulmonary function.
While the potential of HEMT should be celebrated, complications such as IWS must also heighten awareness of the possible deleterious effects of withdrawal. With the increased eligibility for HEMT through elex/tez/iva, the number of patients potentially affected by withdrawal increases as well. Provider-patient communication regarding barriers to adherence is important to mitigate these consequences. Suspicion of this uncommon clinical entity should be considered in CF patients on HEMT with similar presentations.
Ivacaftor redefined the vision of CF treatment and prognosis. Less understood are the equally important, potentially deleterious effects of abrupt withdrawal from this medication.
Dr James Clegg is resident Physician at Nashville Department of Pediatrics, Monroe Carell Jr. Children’s Hospital at Vanderbilt, 2200 Children’s Way, TN 37232, USA.
Patrick A Flume, Reta Fischer Biner, Damian G Downey, Cynthia Brown, Manu Jain, Rainald Fischer, et al and the VX14-661-110 study group. Long-term safety and efficacy of tezacaftor-ivacaftor in individuals with cystic fibrosis aged 12 years or older who are homozygous or heterozygous for Phe508del CFTR (EXTEND): an open-label extension study.Lancet Respir Med 2021 Jul;9(7):733-746.doi: 10.1016/S2213-2600(20)30510-5. Epub 2021 Feb 10. [Pubmed]
Background: Tezacaftor-ivacaftor is an approved cystic fibrosis transmembrane conductance regulator (CFTR) modulator shown to be efficacious and generally safe and well tolerated over 8-24 weeks in phase 3 clinical studies in participants aged 12 years or older with cystic fibrosis homozygous for the Phe508del CFTR mutation (F/F; study 661-106 [EVOLVE]) or heterozygous for the Phe508del CFTR mutation and a residual function mutation (F/RF; study 661-108 [EXPAND]). Longer-term (>24 weeks) safety and efficacy of tezacaftor-ivacaftor has not been assessed in clinical studies. Here, we present results of study 661-110 (EXTEND), a 96-week open-label extension study that assessed long-term safety, tolerability, and efficacy of tezacaftor-ivacaftor in participants aged 12 years or older with cystic fibrosis who were homozygous or heterozygous for the Phe508del CFTR mutation.
Methods: Study 661-110 was a 96-week, phase 3, multicentre, open-label study at 170 clinical research sites in Australia, Europe, Israel, and North America. Participants were aged 12 years or older, had cystic fibrosis, were homozygous or heterozygous for Phe508del CFTR, and completed one of six parent studies of tezacaftor-ivacaftor: studies 661-103, 661-106, 661-107, 661-108, 661-109, and 661-111. Participants received oral tezacaftor 100 mg once daily and oral ivacaftor 150 mg once every 12 h for up to 96 weeks. The primary endpoint was safety and tolerability. Secondary endpoints were changes in lung function, nutritional parameters, and respiratory symptom scores; pulmonary exacerbations; and pharmacokinetic parameters. A post-hoc analysis assessed the rate of lung function decline in F/F participants who received up to 120 weeks of tezacaftor-ivacaftor in studies 661-106 (F/F) and/or 661-110 compared with a matched cohort of CFTR modulator-untreated historical F/F controls from the Cystic Fibrosis Foundation Patient Registry. Primary safety analyses were done in all participants from all six parent studies who received at least one dose of study drug during this study. This study was registered at ClinicalTrials.gov (NCT02565914).
Findings: Between Aug 31, 2015, to May 31, 2019, 1044 participants were enrolled in study 661-110 from the six parent studies of whom 1042 participants received at least one dose of study drug and were included in the safety set. 995 (95%) participants had at least one TEAE; 22 (2%) had TEAEs leading to discontinuation; and 351 (34%) had serious TEAEs. No deaths occurred during the treatment-emergent period; after the treatment-emergent period, two deaths occurred, which were both deemed unrelated to study drug. F/F (106/110; n=459) and F/RF (108/110; n=226) participants beginning tezacaftor-ivacaftor in study 661-110 had improvements in efficacy endpoints consistent with parent studies; improvements in lung function and nutritional parameters and reductions in pulmonary exacerbations observed in the tezacaftor-ivacaftor groups in the parent studies were generally maintained in study 661-110 for an additional 96 weeks. Pharmacokinetic parameters were also similar to those in the parent studies. The annualised rate of lung function decline was 61·5% (95% CI 35·8 to 86·1) lower in tezacaftor-ivacaftor-treated F/F participants versus untreated matched historical controls.
Interpretation: Tezacaftor-ivacaftor was generally safe, well tolerated, and efficacious for up to 120 weeks, and the safety profile of tezacaftor-ivacaftor in study 661-110 was consistent with cystic fibrosis manifestations and with the safety profiles of the parent studies. The rate of lung function decline was significantly reduced in F/F participants, consistent with cystic fibrosis disease modification. Our results support the clinical benefit of long-term tezacaftor-ivacaftor treatment for people aged 12 years or older with cystic fibrosis with F/F or F/RF genotypes.
Patrick O Hanafin, Isabelle Sermet-Gaudelus, Matthias Griese, Matthias Kappler, Helmut Ellemunter, Carsten Schwarz, John Wilson, Marsha Tan, Tony Velkov, Gauri G Rao, Elena K Schneider-Futschik. Insights Into Patient Variability During Ivacaftor-Lumacaftor Therapy in Cystic Fibrosis. Front Pharmacol 2021 Aug 2;12:577263.doi: 10.3389/fphar.2021.577263.eCollection 2021. Free PMC article [Pubmed]

Patrick Hanafin
Background: The advent of cystic fibrosis transmembrane conductance regulator protein (CFTR) modulators like ivacaftor have revolutionised the treatment of cystic fibrosis (CF). However, due to the plethora of variances in disease manifestations in CF, there are inherent challenges in unified responses under CFTR modulator treatment arising from variability in patient outcomes. The pharmacokinetic (PK) data available for ivacaftor-lumacaftor cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulator drug combination is limited.
Methods: Secondary objectives were to identify (1) patient characteristics and (2) the interactions between ivacaftor-lumacaftor responsible for interindividual variability (IIV).
Results: Peak plasma concentrations (Cmax) of ivacaftor – lumacaftor were >10 fold lower than expected compared to label information. The one-way ANOVA indicated that the patient site had an effect on Cmax values of ivacaftor metabolites ivacaftor-M1, ivacaftor-M6, and lumacaftor (p < 0.001, p < 0.001, and p < 0.001, respectively). The Spearman’s rho test indicated that patient weight and age have an effect on the Cmax of lumacaftor (p = 0.003 and p < 0.001, respectively) and ivacaftor metabolite M1 (p = 0.020 and p < 0.001, respectively). Age (p < 0.001) was found to effect on Cmax of ivacaftor M6 and on Tmax of ivacaftor M1 (p = 0.026). A large impact of patient characteristics on the IIV of PK parameters Cmax and Tmax, was observed among the CF patients.
Conclusion: Understanding the many sources of variability can help reduce this individual patient variability and ensure consistent patient outcomes.
Patrick O Hanafin is in the Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States.
Donatello Salvatore, Carmela Colangelo, Michele D’Andria, Giovanni Marsicovetere, Domenica Passarella. Elexacaftor/tezacaftor/ivacaftor as rescue therapy in a patient with the cystic fibrosis genotype F508DEL/ G1244E. Clin Case Rep 2021 Aug 25;9(8):e04713.doi: 10.1002/ccr3.4713.eCollection 2021 Aug. OPEN access for full case reports [Pubmed]

Donatello Salvatore
Elexacaftor/tezacaftor/ivacaftor (ETI) is a cystic fibrosis (CF) transmembrane regulator (CFTR) modulator. It is known to be efficacious in stable patients with severe pneumopathy, but there are few data concerning its effectiveness during acute exacerbations. We here describe its use in a woman with CF and respiratory failure.
A 50-year old woman with severe CF who eventually went into respiratory failure. Our patient had an F/G genotype and had been previously treated with ivacaftor but, after an initial improvement, experienced rapid disease progression (as has been previously described in ivacaftor-treated adults with G mutations), possibly aggravated by Bcc colonization. During her subsequent hospitalization, she did not improve significantly until ETI was administered, and, as all her other treatments remained unchanged, this suggests it played a role in her recovery. Furthermore, her lung disease continued to improve after she was discharged: There was no recurrence of PEx, no need for antibiotics, and her lung function and ABG parameters improved.
Dr Donatello Salvatore is Director of the Cystic Fibrosis Centre – Hospital San Carlo Potenza Italy.
David Drummond, Jérémy Dana, Laureline Berteloot, Elena K Schneider-Futschik, Frédérique Chedevergne, Céline Bailly-Botuha, Thao Nguyen-Khoa, Mathieu Cornet, Muriel Le Bourgeois, Dominique Debray, Muriel Girard, Isabelle Sermet-Gaudelus. Lumacaftor-ivacaftor effects on cystic fibrosis-related liver involvement in adolescents with homozygous F508 del-CFTR. J Cyst Fibros. 2021 Aug 25;S1569-1993(21)01337-0.doi: 10.1016/j.jcf.2021.07.018. Online ahead of print. [Pubmed]

David Drummond
Background: The effects of lumacaftor-ivacaftor on cystic fibrosis transmembrane conductance regulator (CFTR)-associated liver disease remain unclear. The objective of the study was to describe the effect of this treatment on features of liver involvement in a cystic fibrosis (CF) adolescent population homozygous for F508del.
Methods: Clinical characteristics, liver blood tests, abdominal ultrasonography (US), and pancreas and liver proton density fat fraction (PDFF) by magnetic resonance imaging, were obtained at treatment initiation and at 12 months for all patients. Biomarkers of CFTR activity (sweat chloride test, nasal potential difference, and intestinal current measurement) were assessed at initiation and at 6 months therapy.
Results: Of the 37 patients who started ivacaftor/lumacaftor treatment, 28 were eligible for analysis. In this group, before treatment initiation, 4 patients were diagnosed with multinodular liver and portal hypertension, 19 with other forms of CF liver involvement, and 5 with no signs of liver involvement. During treatment, no hepatic adverse reactions were documented, and no patient developed liver failure. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gammaglutamyl transferase (GGT) decreased significantly following initiation of lumacaftor-ivacaftor, and remained so after 12 months treatment. This was not correlated with changes in clinical status, liver and pancreas US and PDFF, fecal elastase, or lumacaftor-ivacaftor serum levels. The most “responsive” patients demonstrated a significant increase in biomarkers of CFTR activity.
Conclusions: These results may suggest a potential beneficial effect of CFTR modulators on CF liver disease and warrant further investigation in larger, prospective studies.
Dr David Drummond is a paediatrician at Service de Pneumologie et Allergologie Pédiatriques, Centre de Référence Maladies Rares Mucoviscidose et Maladies apparentées, Centre de Ressources et de Compétences pour la Mucoviscidose, Hôpital Necker Enfants Malades, Assistance Publique-Hôpitaux de Paris (AP-HP)-Centre, Université de Paris, Paris, France; Université de Paris, Paris, France.
J Crowley, K Croinin, D Mullane, M Ní Chróinín. Restoration of exocrine pancreatic function in child with lumacaftor/ivacaftor therapy in cystic fibrosis. J Cyst Fibros 2021 Sep 9;S1569-1993(21)01373-4.doi: 10.1016/j.jcf.2021.08.032.Online ahead of print. [Pubmed]
Prior to the use of cystic fibrosis (CF) modulator therapy, exocrine pancreatic insufficiency in CF was thought to be irreversible. Ivacaftor therapy has resulted in exocrine pancreatic function restoration in young children and also in older children after more prolonged use. We report a 3 year 6 months old girl with genotype homozygote p.Phe508del diagnosed on newborn screening with cystic fibrosis. She was noted to be pancreatic insufficient at birth and commenced on pancreatic enzyme replacement. Her baseline faecal elastase was 33 ug/g aged 8 months and < 15 ug/g at 22 months with normal reference >200ug/g.
She commenced on orkambi (lumacaftor/ ivacacftor) aged 24 months in February 2020. She was noted to have constipation and reduced enzyme replacement requirements with no symptoms of pancreatic insufficiency. A repeat faecal elastase at 3 years 4 months was 489ug/g showing evidence of restoration of exocrine pancreatic function.
This is the first report of exocrine pancreatic function restoration in a CF patient homozygous for p.Phe508del receiving lumaftor/ ivacaftor therapy. Consideration should be given to repeating faecal elastase in the population of CF children receiving orkambi where symptoms of pancreatic insufficiency have resolved and growth and weight are satisfactory. The prospect of restoration of pancreatic function in this group of young patients is very exciting.
Dr J Crowley is at the Cork University Hospital, Wilton, Cork, Ireland.
Oded Breuer, David Shoseyov, Shifra Koretz, Nadia Alyan, Joel Reiter, Malena Cohen-Cymberknoh, Isaiah Wexler, Eitan Kerem. Ethical dilemma: ELX/TEZ/IVA or Lung Transplantation in Cystic Fibrosis and End Stage Lung Disease? Chest 2021 Sep 7;S0012-3692(21)03846-0.doi: 10.1016/j.chest.2021.08.073.Online ahead of print. [Pubmed]

Oded Breuer
Cystic fibrosis is caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. Novel, highly effective, modulator therapies correcting and potentiating CFTR function are changing the course of the disease. We present an ethical dilemma involving an 11-year-old child with CF and end stage lung disease. Shortly after starting treatment with Elexacaftor-Tezacaftor-Ivacaftor, the family received notification that a matched donor lung had been allocated. Clinical decision-making in this case is challenging as definitive data to medically support one treatment option over the other is limited. A survey of CF center team members was conducted for the purpose of this manuscript. Ethical principles that may guide us in these situations are discussed.
Overall, results of the survey present a lack of agreement as to the best approach in this situation. Physicians, when compared to other team members, are more likely to provide a specific recommendation vs. to present the information and let the family decide (odds ratio (95% confidence interval)=4.0 (1.2-12.8), p=0.021). A shared decision-making model, stressing our moral obligation as clinicians to respect autonomy by appreciating family values while offering to participate in the decision-making process and ensuring non-maleficence, is presented.
In summary, CFTR modulators affect the outcomes of CF disease and influence clinical decision-making. Current lack of data on long-term outcomes, in young patients with CF receiving effective modulator therapy, should not preclude CF team participation in decision-making. Shared decision-making which is focused on respecting autonomy is our preferred approach in these situations.
Emily Granger, Gwyneth Davies, Ruth H Keogh. Treatment patterns in people with cystic fibrosis: have they changed since the introduction of ivacaftor? J Cyst Fibros. 2021 Sep 5;S1569-1993(21)01355-2.doi: 10.1016/j.jcf.2021.08.014.Online ahead of print. 34497037

Emily Granger
Background: In late 2012, ivacaftor became available in the UK for people with cystic fibrosis (CF) aged 6 years and over with a G551D mutation. Long-term changes in treatment patterns have not previously been reported. We investigated long-term treatment patterns in people with CF with a G551D mutation who took ivacaftor and compared these with non-ivacaftor-treated cohorts using the UK Cystic Fibrosis Registry.
Methods: Using 2007-2018 data we compared treatment patterns between four cohorts: 1: ivacaftor-treated; 2: ivacaftor era (2013-2018), ineligible genotype (no G551D mutation); 3: pre-ivacaftor era (2007-2012), eligible genotype (G551D mutation); 4: pre-ivacaftor era, ineligible genotype. Treatments included: inhaled antibiotics, dornase alfa, hypertonic saline, chronic oral antibiotics and supplementary feeding.
Results: Up to 2012 the percentages of people taking each treatment were similar between the two cohorts defined by genotype and tended to increase by year with a similar slope. Once ivacaftor was introduced, the use of other treatments tended to decrease or remain stable by year for the ivacaftor-treated cohort, whereas it remained stable or increased in the non-ivacaftor-treated cohort. This led to differences in treatment use between the two cohorts in the ivacaftor-era, which became more marked over time.
Conclusions: We have shown a clear divergence in treatment patterns since the introduction of ivacaftor in a number of key treatments widely used in CF. Further research is needed to investigate whether the differences in treatment patterns are associated with changes in health outcomes.
Dr Emily Granger is a research fellow in the Department of Medical Statistics, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, Keppel St, Bloomsbury, London.
Donatello Salvatore, Angela Pepe, Vincenzo Carnovale, Fabio Majo, Rita Padoan, Serena Quattrucci, Marco Salvatore, Domenica Taruscio, Annalisa Amato, Gianluca Ferrari, Giuseppe Campagna Elexacaftor/tezacaftor/ivacaftor for CFTR variants giving rise to diagnostic uncertainty: Personalised medicine or over-medicalisation? J Cyst Fibros 2021 Sep 25;S1569-1993(21)01416-8. doi: 10.1016/j.jcf.2021.09.011.Online ahead of print. [Pubmed]
In order to assess the real-world frequency of the three categories that may give rise to diagnostic uncertainty (mutations uncertainly interpreted as being pathogenic, mutations of unknown significance, and non CF-causing variants), we queried the Italian CF Registry (ICFR) and found that 113 (2.06%) of the 5,489 subjects registered and genotyped in 2018 had at least one such mutation: nine had four different mutations uncertainly interpreted as being pathogenic; three had two different mutations of unknown significance; and 101 had ten different non CF-causing variants.
These findings highlight an important question concerning the eligibility of subjects without a clearly diagnostic mutation for CFTR modulator treatment because the number of currently eligi- ble patients has greatly increased and the annual retail cost of ETI exceeds $300,000 per patient, which is much more expensive than the cost of other chronic CF treatments.
On the one hand, CF physicians often have to provide care for patients with rare CF genotypes in clinical practice, and so there is a need to test the potential efficacy of approved modulators in the treatment of rare CF-causing genotypes. On the other, although it is true that robust clinical trial data may be very difficult (if not impossible) to obtain, we wonder whether in vitro data alone are sufficient to justify the use of a very expensive treatment, especially in the case of patients with a diagnosis of CF based on genotypes containing non-CF-causing variants or variants whose clinical effect is doubtful or unknown.
The introduction of second tier CFTR-extended genetic analyses in new-born screening programmes without filtering for all known CF variants with full penetrance would inevitably increase the number of eligible subjects. A significant number of infants with so-called CF transmembrane conductance regulator-related metabolic syndrome (CRMS)/cystic fibrosis screen positive, inconclusive diagnosis (CFSPID) would be considered eligible for HEMT, and their parents would probably expect them to receive it only in the presence of mutations that were simply verifiable on a free- access website. However, the diagnosis of CF is and may remain inconclusive for a long time.
The wise use of economic resources is an important consideration when attempting to improve the access to treatment of patients who could really benefit from HEMT.
Dr Donatello Salvatore is at the Cystic Fibrosis Centre, San Carlo Hospital, Potenza, Italy; Italian Cystic Fibrosis Registry, Rome Italy.
Kevin J Scully, Peter Marchetti, Gregory S Sawicki, Ahmet Uluer, Manuela Cernadas, Rebecca E Cagnina, John C Kennedy, Melissa S Putman. The effect of elexacaftor/tezacaftor/ivacaftor (ETI) on glycemia in adults with cystic fibrosis. J Cyst Fibros 2021 Sep 13;S1569-1993(21)01377-1.doi: 10.1016/j.jcf.2021.09.001.Online ahead of print.[Pubmed]

Kevin Scully
The impact of elexacaftor-tezacaftor-ivacaftor (ETI) on glycemic control is currently unknown. Our objective was to investigate the effect of ETI initiation on glycemia in adults with CF using continuous glucose monitoring (CGM).
Methods: In this prospective observational study, 34 adults with CF and at least one F508del CFTR mutation wore CGM sensors for 14 days prior to starting ETI and again 3-12 months after ETI initiation. Hypoglycemia symptoms were queried at each visit, and most recent anthropometric measures and spirometry data were obtained by chart review.
Results: Twenty-three participants completed the study. Compared to baseline, average glucose (AG), standard deviation (SD), % time >200 mg/dL, and peak sensor glucose decreased with ETI treatment, and % time in target range 70-180 mg/dL increased. Improvements in glycemic parameters were most notable in individuals with CFRD. There was no significant change in CGM-measured or self-reported hypoglycemia before and after ETI initiation.
Conclusion: Initiation of ETI in adults with CF was associated with improvement CGM-derived measures of hyperglycemia and glycemic variability with no effect on hypoglycemia. The authors suggest further studies are needed to investigate underlying etiology of these changes and the long-term impact of ETI on glycemic control in patients with CF.
Dr Kevin J Scully is attending physician and clinical fellow in the Division of Endocrinology, Boston Children’s Hospital, 333 Longwood Avenue, 6th Floor, Boston, MA, 02115, United States; Harvard Medical School, Boston MA, United States.
Rosara Bass, Jefferson N Brownell, Virginia A Stallings. The Impact of Highly Effective CFTR Modulators on Growth and Nutrition Status. Nutrients 2021 Aug 24;13(9):2907. doi: 10.3390/nu13092907 free article [Pubmed]

Rosara Bass
Dysfunctional CFTR contributes to increased energy expenditure, exocrine pancreatic insufficiency causing impaired dietary macronutrient digestion and absorption, intestinal dysbiosis, and impaired bile acid homeostasis. Poor nutritional status as a result of these mechanisms is associated with decreased lung function, worse clinical outcomes, and ultimately, increased mortality. Nutritional interventions addressing these mechanisms, such as pancreatic enzyme-replacement therapy and enteral caloric supplementation, have improved nutritional status and, by association, clinical outcomes. In the last decade, the advent of medications targeting defective CFTR proteins has revolutionized the care of patients with CF by reducing the overall impact of CFTR dysfunction. Below, we summarize the effects of highly effective CFTR modulators on nutritional status overall as well as specific factors including bile acid metabolism, pancreatic function, energy expenditure, and intestinal dysbiosis. The authors suggest the future of CF nutrition care will require a paradigm shift away from focusing on methods addressing CFTR dysfunction such as excess calorie provision and toward an individualized, holistic approach in the context of specific mutations and CFTR-directed therapy.
Table 1 (from full paper available via PubMed) Summary of the effects of highly effective CFTR modulators on markers of growth, nutritional status and gastrointestinal health.
| Outcome | Ivacaftor | ELEX-TEZ-IVA |
| Weight-Z | Increased | Increased |
| Height-Z | Increased in youth | To be determined |
| BMI-Z | Increased | Increased |
| Fat Mass | Increased | To be determined |
| Fat-Free Mass | Unchanged to increased | To be determined |
| Bile Acid Metabolism | Increased FGF-19 and decreased C4 | To be determined |
| CF Liver Disease | To be determined, case reports of improved steatosis | To be determined |
| Exocrine Pancreatic Insufficiency | Increased fecal elastase at younger ages | To be determined |
| Bicarbonate Secretion | Decreased time to reach pH 5.5 | To be determined |
| Energy Expenditure | Decreased | To be determined |
| GI Microbiome | Changes in intestinal flora and decreased calprotectin | To be determined |
Dr Rosara Bass is attending physician with the Division of Gastroenterology, Hepatology and Nutrition at the Children’s Hospital of Philadelphia, 3401 Civic Center Blvd, Philadelphia, PA 19104, USA.
– This is an excellent comprehensive review of the general effects of CFTR modulators on wide variety of factors relevant to nutrition
Ruth H Keogh, Rebecca Cosgriff, Eleni-Rosalina Andrinopoulou, Keith G Brownlee, Siobhán B Carr, Karla Diaz-Ordaz, Emily Granger, Nicholas P Jewell, Alex Lewin, Clemence Leyrat, Daniela K Schlüter, Maarten van Smeden, Rhonda D Szczesniak, Gary J Connett. Projecting the impact of triple CFTR modulator therapy on intravenous antibiotic requirements in cystic fibrosis using patient registry data combined with treatment effects from randomised trials Thorax 2021 Sep 23;thoraxjnl-2020-216265.doi: 10.1136/thoraxjnl-2020-216265.Online ahead of print. [Pubmed]

Ruth Keogh
Background: Cystic fibrosis (CF) is a life-threatening genetic disease, affecting around 10 500 people in the UK. Precision medicines have been developed to treat specific CF-gene mutations. The newest, elexacaftor/tezacaftor/ivacaftor (ELEX/TEZ/IVA), has been found to be highly effective in randomised controlled trials (RCTs) and became available to a large proportion of UK CF patients in 2020. Understanding the potential health economic impacts of ELEX/TEZ/IVA is vital to planning service provision.
Methods: We combined observational UK CF Registry data with RCT results to project the impact of ELEX/TEZ/IVA on total days of intravenous (IV) antibiotic treatment at a population level. Registry data from 2015 to 2017 were used to develop prediction models for IV days over a 1-year period using several predictors, and to estimate 1-year population total IV days based on standards of care pre-ELEX/TEZ/IVA. We considered two approaches to imposing the impact of ELEX/TEZ/IVA on projected outcomes using effect estimates from RCTs: approach 1 based on effect estimates on FEV1% and approach 2 based on effect estimates on exacerbation rate.
Results: ELEX/TEZ/IVA is expected to result in significant reductions in population-level requirements for IV antibiotics of 16.1% (~17 800 days) using approach 1 and 43.6% (~39 500 days) using approach 2. The two approaches require different assumptions. Increased understanding of the mechanisms through which ELEX/TEZ/IVA acts on these outcomes would enable further refinements to our projections.
Conclusions: This work contributes to increased understanding of the changing healthcare needs of people with CF and illustrates how Registry data can be used in combination with RCT evidence to estimate population-level treatment impacts.
Professor Ruth H Keogh is at the Department of Medical Statistics, London School of Hygiene & Tropical Medicine, London, UK
Eline Lauwers, Annemiek Snoeckx , Kris Ides , Kim Van Hoorenbeeck, Maarten Lanclus , Wilfried De Backer, Jan De Backer, Stijn Verhulst. Functional respiratory imaging in relation to classical outcome measures in cystic fibrosis: a cross-sectional study. BMC Pulm Med 2021 Aug 4;21(1):256.doi: 10.1186/s12890-021-01622-3. Free PMC article [Pubmed]

Eline Lauwers
Background: Functional Respiratory Imaging (FRI) combines HRCT scans with computational fluid dynamics to provide objective and quantitative information about lung structure and function. FRI has proven its value in pulmonary diseases such as COPD and asthma, but limited studies have focused on cystic fibrosis (CF). This study aims to investigate the relation of multiple FRI parameters to validated imaging parameters and classical respiratory outcomes in a CF population.
Methods: CF patients aged > 5 years scheduled for a chest CT were recruited in a cross-sectional study. FRI outcomes included regional airway volume, airway wall volume, airway resistance, lobar volume, air trapping and pulmonary blood distribution. Besides FRI, CT scans were independently evaluated by 2 readers using the CF-CT score. Spirometry and the 6-Minute Walk Test (6MWT) were also performed. Statistical tests included linear mixed-effects models, repeated measures correlations, Pearson and Spearman correlations.
Results: 39 CT scans of 24 (17M/7F) subjects were analyzed. Patients were 24 ± 9 years old and had a ppFEV1 of 71 ± 25% at the time of the first CT. All FRI parameters showed significant low-to-moderate correlations with the total CF-CT score, except for lobar volume. When considering the relation between FRI parameters and similar CF-CT subscores, significant correlations were found between parameters related to airway volume, air trapping and airway wall thickening. Air trapping, lobar volume after normal expiration and pulmonary blood distribution showed significant associations with all spirometric parameters and oxygen saturation at the end of 6MWT. In addition, air trapping was the only parameter related to the distance covered during 6MWT. A subgroup analysis showed considerably higher correlations in patients with mild lung disease (ppFEV1 ≥ 70%) compared to patients with moderate to severe lung disease (ppFEV1 < 70%) when comparing FRI to CF-CT scores.
Conclusions: Multiple structural characteristics determined by FRI were associated with abnormalities determined by CF-CT score. Air trapping and pulmonary blood distribution appeared to be the most clinically relevant FRI parameters for CF patients due to their associations with classical outcome measures. The FRI methodology could particularly be of interest for patients with mild lung disease, although this should be confirmed in future research.
Dr Eline Lauwers is a PhD researcher at the Laboratory of Experimental Medicine and Pediatrics, Faculty of Medicine and Health Sciences, University of Antwerp, Universiteitsplein 1, 2160, Wilrijk, Belgium and the Infla-Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium.
Lauryn A Benninger, Cesar Trillo, Jorge Lascano. CFTR modulator use in post lung transplant recipients. J Heart Lung Transplant 2021 Aug 26;S1053-2498(21)02482-7.doi: 10.1016/j.healun.2021.08.009. Online ahead of print. [Pubmed]

Lauryn Benninger
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator therapy has previously been contraindicated in solid organ transplant recipients. This was due to lack of data and concern for interactions with immunosuppressive drug regimens. However, in post-lung transplant recipients, CFTR modulators may improve extrapulmonary manifestations of cystic fibrosis without impacting graft function or immunosuppressive drug levels. Herein, we present our single center experience with the use of elexacaftor/tezacaftor/ivacaftor, Trikafta, in adult post-lung transplant recipients.
Dr Lauryn A Benninger is a pulmonologist in the Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Florida College of Medicine, Gainesville, Florida
Anthony M Kendle, Jared T Roekner, Elsa C Santillana, Lilla E Kis, Mary A Cain. Cystic Fibrosis Transmembrane Conductance Regulator Modulators During Pregnancy: A Case Series. Cureus 2021 Aug 25;13(8):e17427.doi: 10.7759/cureus.17427.eCollection 2021 Aug. Free PMC article [Pubmed]

Anthony M Kendle
We present a series of pregnant patients taking novel CF transmembrane conductance regulator (CFTR) modulators and summarize pertinent clinical considerations. All women conceived within four months after starting elexacaftor-ivacaftor-tezacaftor. Pulmonary function was stable before and during pregnancy. One patient developed transaminitis necessitating discontinuation of the medication mid-trimester.
All patients delivered healthy neonates between 36-38 weeks of gestation with uncomplicated postpartum courses. No birth defects were encountered. Given that newly introduced CFTR modulators may increase fertility among CF patients, contraception counselling, pulmonary function monitoring, liver function monitoring, and multi-disciplinary care are important pillars of management.
Anthony M Kendle is at Department of Obstetrics and Gynaecology, University of South Florida, Tampa, USA.
Philippe Reix, Aurélie Tatopoulos, Iulia Ioan, Muriel Le Bourgeois, Stéphanie Bui, Marie Luce Choukroun, Katia Bessaci-Kabouya, Michele Gerardin, Plamen Bokov, Jennifer Da Silva, Jean-Louis Paillasseur, Pierre Regis Burgel, French Cystic Fibrosis Reference Network study group. Real-world assessment of LCI following lumacaftor-ivacaftor initiation in adolescents and adults with cystic fibrosis. J Cyst Fibros 2021 Jun 25;S1569-1993(21)01286-8.doi: 10.1016/j.jcf.2021.06.002.Online ahead of print. [Pubmed]
Lung clearance index (LCI) is a biomarker of ventilation inhomogeneity. Data are scarce on its usefulness in daily practice for monitoring the effects of treatments in older children and adults with CF. In this French observational study of lumacaftor-ivacaftor, 63 of 845 patients (7.5%) had available LCI performed at baseline and at six (M6; n=34) or 12 months (M12; n=46) after lumacaftor-ivacaftor initiation. At inclusion, median [IQR] age was 16 years [13-17], ppFEV1 was 72.8 [59.6-80.7], and LCI was 12.3 [10.3-15.0].
At both M6 and M12, non statistically significant LCI increases of 0.13 units or 1.34% (95% CI: -4.85-7.53) and 0.6 units or 6.66% (95% CI: -0.03-13.5) were observed. Discordant results between LCI and ppFEV1 were observed in one-third of the patients.
In daily practice, LCI monitoring in adolescents and young adults with moderate lung disease gives results that are more heterogenous than those reported in children with milder disease.
Dr Philippe Reix is at the Cystic Fibrosis Center, Hospices Civils de Lyon, Lyon, France; UMR 5558 CNRS Equipe EMET Université Claude Bernard Lyon 1 Lyon, France.
Edith T Zemanick, Jennifer L Taylor-Cousar, Jane Davies, Ronald L Gibson, Marcus A Mall, Edward F McKone; McNally P; Ramsey BW; Rayment JH; Rowe SM; Tullis E; Ahluwalia N; Chu C; Ho T; Moskowitz SM; Noel S; Tian S; Waltz D; Weinstock TG; Xuan F; Wainwright CE; McColley SA. A Phase 3 Open-Label Study of ELX/TEZ/IVA in Children 6 Through 11 Years of Age With CF and at Least One F508del Allele.Am J Respir Crit Care Med 2021 Mar 18. doi: 10.1164/rccm.202102-0509OC.Online ahead of print.[Pubmed]

Edith Zermanick
Rationale: Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was shown to be efficacious and safe in patients aged 12 years and older with cystic fibrosis and at least one F508del-CFTR allele, but has not been evaluated in children <12 years of age.
Objectives: To assess the safety, pharmacokinetics, and efficacy of ELX/TEZ/IVA in children 6 through 11 years of age with F508del-minimal function or F508del-F508del genotypes.
Methods: In this 24-week open-label Phase 3 study, children (N=66) weighing <30 kg received 50% of the ELX/TEZ/IVA adult daily dose (ELX 100 mg once daily, TEZ 50 mg once daily, and IVA 75 mg every 12 hours) while children weighing ≥30 kg received the full adult daily dose (ELX 200 mg once daily, TEZ 100 mg once daily, and IVA 150 mg every 12 hours).
Measurements and main results: The primary endpoint was safety and tolerability. The safety and pharmacokinetic profiles of ELX/TEZ/IVA were generally consistent with those observed in older patients. The most common reported adverse events (AEs) included cough, headache, and pyrexia; in most of the children who had AEs, these were mild or moderate in severity. Through Week 24, ELX/TEZ/IVA treatment improved the ppFEV1 (10.2 percentage points; 95% confidence interval [CI], 7.9 to 12.6), Cystic Fibrosis Questionnaire-Revised respiratory domain score (7.0 points; 95% CI, 4.7 to 9.2), lung clearance index2.5 (-1.71 units; 95% CI, -2.11 to -1.30), and sweat chloride (-60.9 mmol/L; 95% CI, -63.7 to -58.2); body mass index-for-age z-score increased over the 24-week treatment period compared to the pre-treatment baseline.
Conclusions: Our results show ELX/TEZ/IVA is safe and efficacious in children 6 through 11 years of age with at least one F508del-CFTR allele, supporting its use in this patient population.
Dr Edith T Zemanick is Associate Professor at the Colorado Anschutz Medical Campus and Children’s Hospital Colorado, Department of Pediatrics, Aurora, Colorado, United States
Meghan E McGarry, Susanna A McColley. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol 2021 Jun;56(6):1496-1503.doi: 10.1002/ppul.25285. Epub 2021 Feb 1. [Pubmed]

Megan McGarry
Background: Cystic fibrosis transmembrane conductance regulator (CFTR) modulators are disease-modifying medications for cystic fibrosis (CF) and are shown to be efficacious for only specific CFTR mutations. CFTR mutation frequency varies by ancestry, which is different from but related to demographic racial and ethnic group. Eligibility for CFTR modulator therapy has not been previously reported by race and ethnicity.
Methods: We conducted a cross-sectional study of patients in the 2018 CF Foundation Patient Registry. We analyzed the percentage of patients in each US Census defined racial and ethnic group eligible for CFTR modulators based on CFTR mutations approved by the US FDA and then based on both mutations and FDA approval by age. We compared lung function based on CFTR modulator eligibility and prescription.
Findings: Based on CFTR mutations alone, 92.4% of non-Hispanic White patients, 69.7% of Black/African American patients, 75.6% of Hispanic patients, and 80.5% of other race patients eligible for CFTR modulators. For each CFTR modulator, Black/African American patients were least likely to have eligible mutations, and non-Hispanic White patients were most likely. There was no difference in the disparity between racial and/or ethnic groups with the addition of current FDA approval by age. The lowest pulmonary function in the cohort was seen in non-Hispanic White, Black/African American, and Hispanic patients not eligible for CFTR modulators.
Interpretation: Patients with CF from minority groups are less likely to be eligible for CFTR modulators. Because people with CF who are racial and ethnic minorities have increased disease severity and earlier mortality, this will further contribute to health disparities.
Meghan McGarry is a Pediatric Pulmonologist and Assistant Professor in the Department of Pediatrics, University of California, San Francisco, California, USA.
J Crowley, K Croinin, D Mullane, M Ní Chróinín. Restoration of exocrine pancreatic function in child with lumacaftor/ivacaftor therapy in cystic fibrosis. J Cyst Fibros 2021 Sep 9;S1569-1993(21)01373-4.doi: 10.1016/j.jcf.2021.08.032.Online ahead of print. [Pubmed]

Muireann Ni Chronin
Prior to the use of cystic fibrosis (CF) modulator therapy, exocrine pancreatic insufficiency in CF was thought to be irreversible. Ivacaftor therapy has resulted in exocrine pancreatic function restoration in young children and also in older children after more prolonged use. We report a 3 year 6 months old girl with genotype homozygote p.Phe508del diagnosed on newborn screening with cystic fibrosis. She was noted to be pancreatic insufficient at birth and commenced on pancreatic enzyme replacement. Her baseline faecal elastase was 33 ug/g aged 8 months and < 15 ug/g at 22 months with normal reference >200ug/g.
She commenced on orkambi (lumacaftor/ ivacacftor) aged 24 months in February 2020. She was noted to have constipation and reduced enzyme replacement requirements with no symptoms of pancreatic insufficiency. A repeat faecal elastase at 3 years 4 months was 489ug/g showing evidence of restoration of exocrine pancreatic function.
This is the first report of exocrine pancreatic function restoration in a CF patient homozygous for p.Phe508del receiving lumaftor/ ivacaftor therapy. Consideration should be given to repeating faecal elastase in the population of CF children receiving orkambi where symptoms of pancreatic insufficiency have resolved and growth and weight are satisfactory. The prospect of restoration of pancreatic function in this group of young patients is very exciting.
Dr J Crowley is at the Cork University Hospital, Wilton, Cork, Ireland.
Dr Muireann Ni Chronin is a consultant in Respiratory Medicine at Cork University Hospital
Katharina Habler, Anne-Sophie Kalla, Michael Rychlik, Mathias Bruegel, Daniel Teupser, Susanne Nährig, Michael Vogeser, Michael Paal. Isotope dilution LC-MS/MS quantification of the cystic fibrosis transmembrane conductance regulator (CFTR) modulators ivacaftor, lumacaftor, tezacaftor, elexacaftor, and their major metabolites in human serum. Clin Chem Lab Med 2021 Oct 19.doi: 10.1515/cclm-2021-0724. Online ahead of print [Pubmed]

Katharina Haller
Objectives: Cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators have revolutionized the therapeutic landscape in CF treatment. These vital drugs are extensively metabolized via CYP3A, so caution must be exercised in multimodal CF therapy because of the risk of adverse drug interactions. Our goal was to develop a highly sensitive assay for the purpose of therapeutic drug monitoring (TDM) in diagnostic laboratories
Methods: After protein precipitation, the CFTR modulators ivacaftor, lumacaftor, tezacaftor, elexacaftor, and their metabolites ivacaftor-M1, ivacaftor-M6, and tezacaftor-M1 were separated with a two-dimensional chromatography setup within 5 min, and quantified with stable isotope-labeled internal standards. The method was validated according to the European Medicines Agency (EMA) guideline on bioanalytical method validation and applied to CF patient samples.
Results: Inaccuracy was ≤7.0% and the imprecision coefficient of variation (CV) was ≤8.3% for all quality controls (QCs). The method consistently compensated for matrix effects, recovery, and process efficiency were 105-115 and 96.5-103%, respectively. Analysis of CF serum samples provided concentrations comparable to the pharmacokinetic profile data reported in the EMA assessment report for the triple combination therapy Kaftrio.
Conclusions: We hereby present a robust and highly selective isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) assay for the simultaneous quantification of the so far approved CFTR modulators and their metabolites in human serum. The assay is suitable for state-of-the-art pharmacovigilance of CFTR modulator therapy in CF patients, in order to maximize safety and efficacy, and also to establish dose-response relationships in clinical trials.
Katharina Habler is at the Institute of Laboratory Medicine, University Hospital, LMU Munich, Munich, Germany
Michelle J Gould, Haley Smith, Jonathan H Rayment, Helen Machida, Tanja Gonska, Gary J Galante. CFTR modulators increase risk of acute pancreatitis in pancreatic insufficient patients with cystic fibrosis. J Cyst Fibros 2021 Oct 31;S1569-1993(21)01415-6.doi: 10.1016/j.jcf.2021.09.010.Online ahead of print. [Pubmed]

Michelle Gould
Patients with pancreatic insufficient cystic fibrosis rarely develop acute pancreatitis due to insufficient acinar reserve. We describe a series of five patients under the age of 18 (range 8-16 years) with pancreatic insufficient cystic fibrosis who developed a phenotype in keeping with acute pancreatitis following initiation of CFTR modulator therapy. This occurred at a median of 30 months following CFTR modulator initiation. 3/5 of these patients also developed pancreatic sufficiency or at least an intermediary pancreas status, indicated by fecal elastases above 100 μg/g. This series highlights a mostly unrecognized potential side effect of this therapy as well as the potential of CFTR modulator therapies to improve exocrine pancreatic function, even in adolescent patients.
Michelle J Gould is in the Department of Pediatrics, University of Toronto, Toronto, Ontario, Canada.
Isabelle Fajac , Isabelle Sermet. Therapeutic Approaches for Patients with Cystic Fibrosis Not Eligible for Current CFTR Modulators. Cells 2021 Oct 19;10(10):2793.doi: 10.3390/cells10102793. Free PMC article [Pubmed]

Isabelle Fajac
Cystic fibrosis is a severe autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene encoding the CFTR protein, a chloride channel expressed in many epithelial cells. New drugs called CFTR modulators aim at restoring the CFTR protein function, and they will benefit many patients with cystic fibrosis in the near future.
However, some patients bear rare mutations that are not yet eligible for CFTR modulators, although they might be amenable to these new disease-modifying drugs. Moreover, more than 10% of CFTR mutations do not produce any CFTR protein for CFTR modulators to act upon.
The purpose of this review is to provide an overview of different approaches pursued to treat patients bearing mutations ineligible for CFTR modulators. One approach is to broaden the numbers of mutations eligible for CFTR modulators. This requires developing strategies to evaluate drugs in populations bearing very rare genotypes. Other approaches aiming at correcting the CFTR defect develop new mutation-specific or mutation-agnostic therapies for mutations that do not produce a CFTR protein: readthrough agents for nonsense mutations, nucleic acid-based therapies, RNA- or DNA-based, and cell-based therapies. Most of these approaches are in pre-clinical development or, for some of them, early clinical phases. Many hurdles and challenges will have to be solved before they can be safely translated to patients.
Prof. Isabelle Fajac is at the Physiology Department at the AP-HP. Centre-Université de Paris, Hôpital Cochin, Centre de Référence Maladie Rare-Mucoviscidose, 75014 Paris, France and the Faculté de Médecine, Université de Paris, 75006 Paris, France.
Rebecca H Goldberg, Natalie H Matthews, Alexandra C Hristov, Frank Wang. Urticaria multiforme-like eruption due to a novel agent elexacaftor/tezacaftor/ivacaftor in a pediatric patient with cystic fibrosis. JAAD Case Rep. 2021 Oct 25;18:71-73.doi: 10.1016/j.jdcr.2021.10.018.eCollection 2021 Dec.Free article [Pubmed] 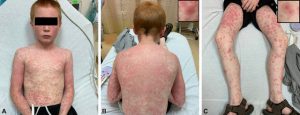

Rebecca Goldberg
Rebecca H Goldberg is at the University of Michigan Medical School, Ann Arbor, Michigan.
(Please note there is a free article with good quality images via PubMed)
Mahan Salehi , Mubashar Iqbal, Asha Dube, Amer AlJoudeh, Frank Edenborough.Delayed hepatic necrosis in a cystic fibrosis patient taking Elexacaftor/Tezacaftor/Ivacaftor (Kaftrio)Respir Med Case Rep 2021 Nov 10;34:101553.doi: 10.1016/j.rmcr.2021.101553. eCollection 2021 Free PMC article. [Pubmed]

Frank Edenborough

Mahan Salehi
The introduction of Cystic Fibrosis Trans Regulatory modulator (CFTRm) drugs has seen a transformation in Cystic Fibrosis (CF) treatment. This has led to a significant improvement in lung function and quality of life with the potential for a real impact on life expectancy. Transient mild to moderate hepatic transaminitis is a well-known side effect of CFTRm drugs, which often improves on cessation and may not recur following the re-institution of the drug. We describe a case of transaminitis developing nine months after the initiation of Kaftrio, which progressed to liver necrosis despite stopping Kaftrio and took many months to resolve. The patient had experienced significant improvement in lung function and overall health while on Kaftrio and deteriorated when it was stopped. He was keen to restart; however, Kaftrio was not reinstated due to the potential risk of acute liver failure.
Mahan Salehi an Academic Foundation Doctor at the Sheffield Teaching Hospitals, Adult Cystic Fibrosis Centre, Sheffield, UK.
Lo M Sosinski, Christian Martin H, Kerri A Neugebauer, Lydia-Ann J Ghuneim, Douglas V Guzior, Alicia Castillo-Bahena, Jenna Mielke, Ryan Thomas, Marc McClelland, Doug Conrad, Robert A Quinn. A restructuring of microbiome niche space is associated with Elexacaftor-Tezacaftor-Ivacaftor therapy in the cystic fibrosis lung J Cyst Fibros 2021 Nov 22;S1569-1993(21)02131-7.doi: 10.1016/j.jcf.2021.11.003.Online ahead of print. [Pubmed]

Lo Sosinski
Background: Elexacaftor-Tezacaftor-Ivacaftor (ETI) therapy is showing promising efficacy for treatment of cystic fibrosis (CF) and is becoming more widely available since recent FDA approval. However, little is known about how these drugs will affect lung infections, which are the leading cause of morbidity and mortality among people with CF (pwCF).
Methods: We analyzed sputum microbiome and metabolome data from pwCF (n=24) before and after ETI therapy using 16S rRNA gene sequencing and untargeted metabolomics.
Results: The sputum microbiome diversity, particularly its evenness, was increased (p=0.036) and the microbiome profiles were different between individuals before and after therapy (PERMANOVA F=1.92, p=0.044). Despite these changes, the microbiomes remained more similar within an individual than across the sampled population. No specific microbial taxa differed in relative abundance before and after therapy, but the collective log-ratio of classic CF pathogens to anaerobes significantly decreased (p=0.013). The sputum metabolome also showed changes associated with ETI (PERMANOVA F=4.22, p=0.002) and was characterized by greater variation across subjects while on treatment. Changes in the metabolome were driven by a decrease in peptides, amino acids, and metabolites from the kynurenine pathway, which were associated with a decrease in CF pathogens. Metabolism of the three small molecules that make up ETI was extensive, including previously uncharacterized structural modifications.
Conclusions: ETI therapy is associated with a changing microbiome and metabolome in airway mucus. This effect was stronger on sputum biochemistry, which may reflect changing niche space for microbial residency in lung mucus as the drug’s effects take hold.
Lo M Sosinski is a graduate student in the Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI, USA
Jérémy Dana, Dominique Debray ,Aurélie Beaufrère, Sophie Hillaire , Monique Fabre Caroline Reinhold, Thomas Baumert, Laureline Berteloot, Valérie Vilgrain. Cystic fibrosis-related liver disease: clinical presentations, diagnostic and monitoring approaches in the era of CFTR modulator therapies. J Hepatol 2021 Oct 19;S0168-8278(21)02115-2 doi: 10.1016/j.jhep.2021.09.042.Online ahead of print. [Pubmed]
Cystic fibrosis (CF) is the most common autosomal recessive disease in the Caucasian population. Cystic Fibrosis-related Liver Disease (CFLD) is defined by the pathogenesis related to the underlying CFTR defect in biliary epithelial cells. CFLD needs to be distinguished from other liver manifestations that may not have any pathological significance. The clinical and histological presentation as well as the severity of CFLD varies. The main histological presentation of CFLD is focal biliary fibrosis, which is usually asymptomatic. Portal hypertension develops in a minority of cases (about 10%) and may require specific management including liver transplantation for end-stage liver disease. Portal hypertension is usually the result of the progression of focal biliary fibrosis to multilobular cirrhosis during childhood. Nevertheless, non-cirrhotic portal hypertension as a result of porto-sinusoidal vascular disease is now identified increasingly more frequently in these patients, mainly in young adults. To evaluate the effect of new CFTR modulator therapies on the liver, the spectrum of hepatobiliary involvement must first be precisely classified. This paper discusses the phenotypical features of CFLD, its underlying physiopathology and relevant diagnostic and follow-up approaches with a special focus on imaging.
Jérémy Dana is in the Department of Radiology, Beaujon Hospital, Université de Paris, Clichy, France; Université de Strasbourg, Strasbourg, France; Inserm, U1110, Institut de Recherche sur Les Maladies Virales et Hépatiques, Strasbourg, France; IHU-Strasbourg (Institut Hospitalo-Universitaire), Pôle Hépato-digestif, Nouvel Hôpital Civil, Strasbourg, France.
David P Nichols, Alex C Paynter, Sonya L Heltshe, Scott H Donaldson, Carla A Frederick, Steven D Freedman et al, PROMISE Study Group. Clinical Effectiveness of Elexacaftor/Tezacftor/Ivacaftor in People with Cystic Fibrosis Am J Respir Crit Care Med 2021 Nov 16.doi: 10.1164/rccm.202108-1986OC.Online ahead of print.[Pubmed]

David Nichols
Rationale: The cystic fibrosis (CF) modulator drug elexacaftor/tezacaftor/ivacaftor (ETI) proved highly effective in controlled clinical trials for individuals with ≥1 F508del allele, which occurs in at least 85% of people with CF (PwCF).
Objective: PROMISE is a post-approval study to understand the broad effects of ETI through 30 months clinical use in a more diverse US patient population with planned analyses after 6 months
Methods: Prospective, observational study in 487 PwCF age ≥12 years with ≥1 F508del allele starting ETI for the first time. Assessments occurred before and 1, 3, and 6 months into ETI therapy. Outcomes included change in ppFEV1, sweat chloride concentration, body mass index, and self-reported respiratory symptoms.
Results: average age was 25.1 years. 44.1% entered the study using tezacaftor/ivacaftor or lumacaftor/ivacaftor while 6.7% were using ivacaftor, consistent with F508del homozygosity and G551D allele, respectively. At 6 months into ETI therapy, ppFEV1 improved 9.76 percentage points (95% CI 8.76, 10.76) from baseline, CFQ-R Respiratory Domain score improved 20.4 points (95% CI 18.3, 22.5), and sweat chloride decreased -41.7 mmol/L (95% CI 43.8, 39.6). BMI also significantly increased. Changes were larger in those naïve to modulators but substantial in all groups, including those treated with ivacaftor at baseline.
Conclusions: ETI by clinical prescription provided large improvements in lung function, respiratory symptoms, and BMI in a diverse population naïve to modulator drug therapy, using existing two-drug combinations, or using ivacaftor alone. Each group also experienced significant reductions in sweat chloride concentration, which correlated with improved ppFEV1 in the overall study population.
David P Nichols is in the University of Washington School of Medicine, 12353, Pediatrics, Seattle, Washington, United States.Seattle Children’s Hospital, 7274, Pediatric Pulmonology, Seattle, Washington, United States.
Michael Williamson, Michelle Casey, Claudie Gabillard-Lefort, Aram Alharbi, Yu Qing Jolene Teo, Noel G McElvaney, Emer Reeves.Current evidence on the effect of highly effective CFTR modulation on interleukin-8 in cystic fibrosis. Expert Rev Respir Med 2021 Nov 2.doi: 10.1080/17476348.2021.2001333.Online ahead of print.[Pubmed]
Introduction:Cystic fibrosis (CF) is a genetically inherited disease, with mortality and morbidity associated with respiratory disease. The inflammatory response in CF is characterized by excessive neutrophil influx to the airways, mainly due to the increased local production and retention of interleukin-8 (IL-8), a potent neutrophil chemoattractant.
Areas covered:: We discuss how the chemokine IL-8 more than any other dominates the inflammatory profile of the airways in CF lung disease. Cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies are designed to correct the malfunctioning protein resulting from specific CFTR mutations. This review covers current evidence on the impact of CFTR impairment on levels of IL-8 and outlines the influence of effective CFTR modulation on inflammation in CF with a focus on cytokine production. Review of the literature was carried out using the PUBMED database, Google Scholar and The Cochrane Library databases, using several appropriate generic terms.
Expert opinion:: Therapeutic interventions specifically targeting the defective CFTR protein have improved the outlook for CF. Accumulating studies on the effect of highly effective CFTR modulation on inflammation indicate an impact on IL-8 levels. Further studies are required to increase our knowledge of early onset innate inflammatory dysregulation and on anti-inflammatory mechanisms of CFTR modulators.
Michael Williamson is Consultant Respiratory Physician is at the Royal College of Surgeons in Ireland, Irish Centre for Genetic Lung Disease, Department of Medicine, Royal College of Surgeons in Ireland, Beaumont Hospital, Dublin, Ireland
Max C Petersen, Lauren Begnel, Michael Wallendorf, Marina Litvin. Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J Cyst Fibros 2021 Nov 30;S1569-1993(21)02153-6.doi: 10.1016/j.jcf.2021.11.012.Online ahead of print. [Pubmed]

Max Petersen
Background:Though weight gain has been reported in some clinical trials of CFTR modulators, the effect of elexacaftor-tezacaftor-ivacaftor on body weight, body mass index (BMI), blood pressure, lipids and glycemic control in the real-world setting remains incompletely described.
Methods:We performed a single-center, retrospective, observational analysis of the effect of elexacaftor-tezacaftor-ivacaftor on body weight and cardiometabolic parameters in 134 adult CF patients of the Washington University Adult Cystic Fibrosis Center. Body weight, BMI, and blood pressure were extracted from outpatient clinic visits for the year preceding and the period following the initiation of elexacaftor-tezacaftor-ivacaftor. Other metabolic parameters were extracted at baseline and at latest available follow-up.
Results:A mean of 12.2 months of follow-up data was available for analysis. The mean rate of change in BMI was 1.47 kg/m2/yr (95% CI, 1.08 to 1.87) greater after initiation of elexacaftor-tezacaftor-ivacaftor. Significant increases in blood pressure were observed. In those without CFRD, random blood glucose and hemoglobin A1c were decreased after elexacaftor-tezacaftor-ivacaftor initiation. In those with CFRD, elexacaftor-tezacaftor-ivacaftor increased serum total cholesterol, HDL-cholesterol, and LDL-cholesterol.
Conclusions:In this single-center, retrospective, observational study of 134 adults with CF, initiation of elexacaftor-tezacaftor-ivacaftor was associated with increases in BMI at a mean follow up of 12.2 months. Changes in other cardiometabolic risk factors were also observed. Widespread use of elexacaftor-tezacaftor-ivacaftor may be expected to increase the incidence of overnutrition in the CF population.
Max C Petersen is in the Division of Endocrinology, Metabolism, & Lipid Research, Department of Medicine, Washington University, St. Louis, Missouri, USA.
Peter J Barry, Marcus A Mall, Antonio Álvarez1, Carla Colombo, Karin M de Winter-de Groot, Isabelle Fajac et al. VX18-445-104 Study Group Collaborators, Triple Therapy for Cystic Fibrosis Phe508del-Gating and -Residual Function Genotypes. N Engl J Med 2021 Aug 26;385(9):815-825.doi: 10.1056/NEJMoa2100665. [Pubmed]

Peter J Barry
Background: Elexacaftor-tezacaftor-ivacaftor is a small-molecule cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimen shown to be efficacious in patients with at least one Phe508del allele, which indicates that this combination can modulate a single Phe508delallele. In patients whose other CFTR allele contains a gating or residual function mutation that is already effectively treated with previous CFTR modulators (ivacaftor or tezacaftor-ivacaftor), the potential for additional benefit from restoring Phe508del CFTR protein function is unclear.
Methods: We conducted a phase 3, double-blind, randomized, active-controlled trial involving patients 12 years of age or older with cystic fibrosis and Phe508del-gating or Phe508del-residual function genotypes. After a 4-week run-in period with ivacaftor or tezacaftor-ivacaftor, patients were randomly assigned to receive elexacaftor-tezacaftor-ivacaftor or active control for 8 weeks. The primary end point was the absolute change in the percentage of predicted forced expiratory volume in 1 second (FEV1) from baseline through week 8 in the elexacaftor-tezacaftor-ivacaftor group
Results: After the run-in period, 132 patients received elexacaftor-tezacaftor-ivacaftor and 126 received active control. Elexacaftor-tezacaftor-ivacaftor resulted in a percentage of predicted FEV1 that was higher by 3.7 percentage points (95% confidence interval [CI], 2.8 to 4.6) relative to baseline and higher by 3.5 percentage points (95% CI, 2.2 to 4.7) relative to active control and a sweat chloride concentration that was lower by 22.3 mmol per liter (95% CI, 20.2 to 24.5) relative to baseline and lower by 23.1 mmol per liter (95% CI, 20.1 to 26.1) relative to active control (P<0.001 for all comparisons). The change from baseline in the Cystic Fibrosis Questionnaire-Revised respiratory domain score (range, 0 to 100, with higher scores indicating better quality of life) with elexacaftor-tezacaftor-ivacaftor was 10.3 points (95% CI, 8.0 to 12.7) and with active control was 1.6 points (95% CI, -0.8 to 4.1). The incidence of adverse events was similar in the two groups; adverse events led to treatment discontinuation in one patient (elevated aminotransferase level) in the elexacaftor-tezacaftor-ivacaftor group and in two patients (anxiety or depression and pulmonary exacerbation) in the active control group.
Conclusions: Elexacaftor-tezacaftor-ivacaftor was efficacious and safe in patients with Phe508del-gating or Phe508del-residual function genotypes and conferred additional benefit relative to previous CFTR modulators. (Funded by Vertex Pharmaceuticals; VX18-445-104 ClinicalTrials.gov number, NCT04058353.).
Dr Peter J Barry is at the Manchester University NHS Foundation Trust, Manchester, United Kingdom.
Cori L Daines, Wayne J Morgan The Future of Highly Effective Modulator Therapy in Cystic Fibrosis.. Am J Respir Crit Care Med 2021 Jun 15;203(12):1453-1455.doi: 10.1164/rccm.202104-0850ED. Free PMC article [Pubmed]

Wayne J Morgan

Cori Daines
There is no abstract, but the Free PMC article is a clear and recommended commentary on the article of Zemanick ET et al, Am J Respir Crit Care Med 2021;203:1522-1532. The other important references in this article are all abstracted in the appropriate date sections of this history.
The success of highly effective modulator therapy (HEMT) in cystic fibrosis (CF) now illustrates two areas of deficiency: the lack of HEMT for younger children and for approximately 10% of the CF population without a qualifying mutation.
The article concludes – At this point, the previous modulators IVA, lumacaftor/IVA, and TEZ/IVA have all been approved for younger ages than the original phase III qualifying studies IVA has recently been approved down to 4 months, whereas lumacaftor/IVA is approved to 2 years and TEZ/IVA is approved to 6 years. Longer-term data from these extensions show ongoing safety and efficacy even in the youngest patients. Previous modulators have been shown to be safe, both in the short and long term, in young children. This knowledge should reaffirm the safety findings in the current study and should prove the appropriateness of moving ELX/TEZ/IVA to the 6- to 11-year-old age group. The CF community has been waiting for the results of this study and to have ELX/TEZ/IVA for children. The goal and expectation will be much better long-term outcomes for people living with CF.
Both authors are in the Department of Pediatrics University of Arizona Tucson, Arizona.
Cori L Daines is a professor of Pediatric Pulmonary Medicine
Wayne J Morgan is Professor of Pediatrics and Physiology Arizona Elks Foundation Chair for Statewide Pediatric Research, Program Director, Pediatric Pulmonary Fellowship Pediatric Pulmonary and Sleep Medicine
Edith T Zemanick, Jennifer L Taylor-Cousar, Jane Davies, Ronald L Gibson, Marcus A Mall, Edward F McKone; McNally P; Ramsey BW; Rayment JH; Rowe SM; Tullis E; Ahluwalia N; Chu C; Ho T; Moskowitz SM; Noel S; Tian S; Waltz D; Weinstock TG; Xuan F; Wainwright CE; McColley SA. A Phase 3 Open-Label Study of ELX/TEZ/IVA in Children 6 Through 11 Years of Age With CF and at Least One F508del Allele.Am J Respir Crit Care Med 2021 Mar 18. doi: 10.1164/rccm.202102-0509OC.Online ahead of print.[Pubmed]

Edith Zermanick
Rationale: Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was shown to be efficacious and safe in patients aged 12 years and older with cystic fibrosis and at least one F508del-CFTR allele, but has not been evaluated in children <12 years of age.
Objectives: To assess the safety, pharmacokinetics, and efficacy of ELX/TEZ/IVA in children 6 through 11 years of age with F508del-minimal function or F508del-F508del genotypes.
Methods: In this 24-week open-label Phase 3 study, children (N=66) weighing <30 kg received 50% of the ELX/TEZ/IVA adult daily dose (ELX 100 mg once daily, TEZ 50 mg once daily, and IVA 75 mg every 12 hours) while children weighing ≥30 kg received the full adult daily dose (ELX 200 mg once daily, TEZ 100 mg once daily, and IVA 150 mg every 12 hours).
Measurements and main results: The primary endpoint was safety and tolerability. The safety and pharmacokinetic profiles of ELX/TEZ/IVA were generally consistent with those observed in older patients. The most common reported adverse events (AEs) included cough, headache, and pyrexia; in most of the children who had AEs, these were mild or moderate in severity. Through Week 24, ELX/TEZ/IVA treatment improved the ppFEV1 (10.2 percentage points; 95% confidence interval [CI], 7.9 to 12.6), Cystic Fibrosis Questionnaire-Revised respiratory domain score (7.0 points; 95% CI, 4.7 to 9.2), lung clearance index2.5 (-1.71 units; 95% CI, -2.11 to -1.30), and sweat chloride (-60.9 mmol/L; 95% CI, -63.7 to -58.2); body mass index-for-age z-score increased over the 24-week treatment period compared to the pre-treatment baseline.
Conclusions: Our results show ELX/TEZ/IVA is safe and efficacious in children 6 through 11 years of age with at least one F508del-CFTR allele, supporting its use in this patient population.
Dr Edith T Zemanick is Associate Professor at the Colorado Anschutz Medical Campus and Children’s Hospital Colorado, Department of Pediatrics, Aurora, Colorado, United States
Jia Liu, Allison P Berg, Yiting Wang, Walailak Jantarajit , Katy J Sutcliffe, Edward B Stevens, Lishuang Cao, Marko J Pregel, David N Sheppard. A small molecule CFTR potentiator restores ATP-dependent channel gating to the cystic fibrosis mutant G551D-CFTR. Br J Pharmacol 2021 Oct 13.doi: 10.1111/bph.15709. Online ahead of print. [Pubmed]

David Sheppard
Background and purpose: Cystic fibrosis transmembrane conductance regulator (CFTR) potentiators are small molecules developed to treat the genetic disease cystic fibrosis (CF). They interact directly with CFTR Cl– channels at the plasma membrane to enhance channel gating. Here, we investigate the action of a new CFTR potentiator, CP-628006 with a distinct chemical structure.
Experimental approach: Using electrophysiological assays with CFTR-expressing heterologous cells and CF patient-derived human bronchial epithelial (hBE) cells, we compared the effects of CP-628006 with the marketed CFTR potentiator ivacaftor.
Key results: CP-628006 efficaciously potentiated CFTR function in epithelia from cultured hBE cells. Its effects on the predominant CFTR variant F508del-CFTR were larger than those with the gating variant G551D-CFTR. In excised inside-out membrane patches, CP-628006 potentiated wild-type, F508del- and G551D-CFTR by increasing the frequency and duration of channel openings. CP-628006 increased the affinity and efficacy of F508del-CFTR gating by ATP. In these respects, CP-628006 behaved like ivacaftor. CP-628006 also demonstrated notable differences with ivacaftor. Its potency and efficacy were lower than those of ivacaftor. CP-628006 conferred ATP-dependent gating on G551D-CFTR, whereas the action of ivacaftor was ATP-independent. For G551D-CFTR, but not F508del-CFTR, the action of CP-628006 plus ivacaftor was greater than ivacaftor alone. CP-628006 delayed, but did not prevent, the deactivation of F508del-CFTR at the plasma membrane, whereas ivacaftor accentuated F508del-CFTR deactivation
Conclusions and implications: CP-628006 has distinct effects compared to ivacaftor, suggesting a different mechanism of CFTR potentiation. The emergence of CFTR potentiators with diverse modes of action makes therapy with combinations of potentiators a possibility.
Jla Liu is at the Neuroscience and Pain Research Unit, Pfizer Inc., Granta Park, Great Abington, Cambridge, UK. and the School of Physiology, Pharmacology Neuroscience, University of Bristol, Biomedical Sciences Building, University Walk, Bristol, UK.
Professor David Sheppard is professor in the School of Physiology and Pharmacology University of Bristol and his group have been involved with basic CF research for many years.
Manuel Manfred Nietert, Liza Vinhoven, Florian Auer, Sylvia Hafkemeyer, Frauke Stanke. Comprehensive Analysis of Chemical Structures That Have Been Tested as CFTR Activating Substances in a Publicly Available Database CandActCFTR. Front Pharmacol 2021 Dec 8;12:689205.doi: 10.3389/fphar.2021.689205. eCollection 2021.Free PMC article [Pubmed]

Manuel M Nietert
Background: Cystic fibrosis (CF) is a genetic disease caused by mutations in CFTR, which encodes a chloride and bicarbonate transporter expressed in exocrine epithelia throughout the body. Recently, some therapeutics became available that directly target dysfunctional CFTR, yet research for more effective substances is ongoing. The database CandActCFTR aims to provide detailed and comprehensive information on candidate therapeutics for the activation of CFTR-mediated ion conductance aiding systems-biology approaches to identify substances that will synergistically activate CFTR-mediated ion conductance based on published data.
Results: Until 10/2020, we derived data from 108 publications on 3,109 CFTR-relevant substances via the literature database PubMed and further 666 substances via ChEMBL; only 19 substances were shared between these sources. One hundred and forty-five molecules do not have a corresponding entry in PubChem or ChemSpider, which indicates that there currently is no single comprehensive database on chemical substances in the public domain. Apart from basic data on all compounds, we have visualized the chemical space derived from their chemical descriptors via a principal component analysis annotated for CFTR-relevant biological categories. Our online query tools enable the search for most similar compounds and provide the relevant annotations in a structured way. The integration of the KNIME software environment in the back-end facilitates a fast and user-friendly maintenance of the provided data sets and a quick extension with new functionalities, e.g., new analysis routines. CandActBase automatically integrates information from other online sources, such as synonyms from PubChem and provides links to other resources like ChEMBL or the source publications.
Conclusion: CandActCFTR aims to establish a database model of candidate cystic fibrosis therapeutics for the activation of CFTR-mediated ion conductance to merge data from publicly available sources. Using CandActBase, our strategy to represent data from several internet resources in a merged and organized form can also be applied to other use cases. For substances tested as CFTR activating compounds, the search function allows users to check if a specific compound or a closely related substance was already tested in the CF field. The acquired information on tested substances will assist in the identification of the most promising candidates for future therapeutics.
Dr Manuel Manfred Nietert is Group Leader in the Department of Medical Bioinformatics, University Medical Center Göttingen, Göttingen, Germany. CIDAS Campus Institute Data Science, Georg-August-University, Göttingen, Germany.
Carlos M Farinha, Martina Gentzsch. Revisiting CFTR Interactions: Old Partners and New Players. Int J Mol Sci 2021 Dec 7;22(24):13196.doi:10.3390/ijms222413196. [Pubmed] Free PMC article

Carlos Farinha
Remarkable progress in CFTR research has led to the therapeutic development of modulators that rescue the basic defect in cystic fibrosis. There is continuous interest in studying CFTR molecular disease mechanisms as not all cystic fibrosis patients have a therapeutic option available. Addressing the basis of the problem by comprehensively understanding the critical molecular associations of CFTR interactions remains key. With the availability of CFTR modulators, there is interest in comprehending which interactions are critical to rescue CFTR and which are altered by modulators or CFTR mutations. Here, the current knowledge on interactions that govern CFTR folding, processing, and stability is summarized. Furthermore, we describe protein complexes and signal pathways that modulate the CFTR function. Primary epithelial cells display a spatial control of the CFTR interactions and have become a common system for preclinical and personalized medicine studies. Strikingly, the novel roles of CFTR in development and differentiation have been recently uncovered and it has been revealed that specific CFTR gene interactions also play an important role in transcriptional regulation. For a comprehensive understanding of the molecular environment of CFTR, it is important to consider CFTR mutation-dependent interactions as well as factors affecting the CFTR interactome on the cell type, tissue-specific, and transcriptional levels.
Carlos M Farinha is Associate Professor at BioISI-Biosystems and Integrative Sciences Institute, Faculty of Sciences, University of Lisboa, 1749-016 Lisboa, Portugal.
Sivagurunathan Sutharsan, Edward F McKone, Damian G Downey, Jamie Duckers, Gordon MacGregor, Elizabeth Tullis, Eva Van Braeckel, Claire E Wainwright, Danie Watson, Neil Ahluwalia, Bote G Bruinsma, Christopher Harris, Anna P Lam, Yiyue Lou, Samuel M Moskowitz, Simon Tian, Jason Yuan, David Waltz, Marcus A Mall, VX18-445-109 study group. Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir Med 2021 Dec 20;S2213-2600(21)00454-9.doi: 10.1016/S2213-2600(21)00454-9. Online ahead of print [Pubmed]

Sivagurunathan-Sutharsan
Background:Elexacaftor plus tezacaftor plus ivacaftor is a triple-combination cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimen shown to be generally safe and efficacious in people with cystic fibrosis aged 12 years or older with at least one F508del-CFTR allele. We aimed to assess the magnitude and durability of the clinical effects of this triple combination regimen in people with cystic fibrosis homozygous for the F508del-CFTR mutation.
Methods:We conducted a multicentre, randomised, double-blind, active-controlled, phase 3b trial of elexacaftor plus tezacaftor plus ivacaftor at 35 medical centres in Australia, Belgium, Germany, and the UK. Eligible participants were those with cystic fibrosis homozygous for the F508del-CFTR mutation, aged 12 years or older with stable disease, and with a percent predicted FEV1 of 40-90% inclusive. After a 4-week run-in period, in which participants received tezacaftor 100 mg orally once daily and ivacaftor 150 mg orally every 12 h, participants were randomly assigned (1:1) to receive 24 weeks of either elexacaftor 200 mg orally once daily plus tezacaftor 100 mg orally once daily plus ivacaftor 150 mg orally every 12 h (elexacaftor plus tezacaftor plus ivacaftor group) or tezacaftor 100 mg orally once daily plus ivacaftor 150 mg orally every 12 h (tezacaftor plus ivacaftor group). Randomisation was stratified by percent predicted FEV1, age at screening visit, and whether the participant was receiving CFTR modulators at the time of the screening visit. Patients, investigators, and sponsor’s study execution team were masked to treatment assignment. The primary endpoint was the absolute change in Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score from baseline (ie, at the end of the tezacaftor plus ivacaftor run-in period) up to and including week 24. The key secondary endpoint was the absolute change from baseline in percent predicted FEV1 up to and including week 24; other secondary endpoints were the absolute change from baseline in sweat chloride concentrations up to and including week 24, and safety and tolerability. All endpoints were assessed in all randomised patients who had received at least one dose of their assigned regimen. This study is registered with ClinicalTrials.gov, NCT04105972.
Findings:Between Oct 3, 2019, and July 24, 2020, 176 participants were enrolled. Following the 4-week tezacaftor plus ivacaftor run-in period, 175 participants were randomly assigned (87 to the elexacaftor plus tezacaftor plus ivacaftor group and 88 to the tezacaftor plus ivacaftor group) and dosed in the treatment period. From baseline up to and including week 24, the mean CFQ-R respiratory domain score increased by 17·1 points (95% CI 14·1 to 20·1) in the elexacaftor plus tezacaftor plus ivacaftor group and by 1·2 points (-1·7 to 4·2) in the tezacaftor plus ivacaftor group (least squares mean treatment difference 15·9 points [95% CI 11·7 to 20·1], p<0·0001), the mean percent predicted FEV1 increased by 11·2 percentage points (95% CI 9·8 to 12·6) in the elexacaftor plus tezacaftor plus ivacaftor group and by 1·0 percentage points (-0·4 to 2·4) in the tezacaftor plus ivacaftor group (least squares mean treatment difference 10·2 percentage points [8·2 to 12·1], p<0·0001), and the mean sweat chloride concentration decreased by 46·2 mmol/L (95% CI 43·7 to 48·7) in the elexacaftor plus tezacaftor plus ivacaftor group and by 3·4 mmol/L (1·0 to 5·8) in the tezacaftor plus ivacaftor group (least squares mean treatment difference -42·8 mmol/L [-46·2 to -39·3], nominal p<0·0001). Most participants (70 [80%] in the elexacaftor plus tezacaftor plus ivacaftor group and 74 [84%] in the tezacaftor plus ivacaftor group) had adverse events that were mild or moderate in severity; serious adverse events occurred in five (6%) of 87 participants in the elexacaftor plus tezacaftor plus ivacaftor group and 14 (16%) of 88 participants in the tezacaftor plus ivacaftor group. One (1%) participant in the elexacaftor plus tezacaftor plus ivacaftor group discontinued treatment due to an adverse event of anxiety and depression. Two (2%) participants in the tezacaftor plus ivacaftor group discontinued treatment due to adverse events of psychotic disorder (n=1) and obsessive-compulsive disorder (n=1).
Interpretation:The elexacaftor plus tezacaftor plus ivacaftor regimen was safe and well tolerated, and led to significant and clinically meaningful improvements in respiratory-related quality of life and lung function, as well as improved CFTR function, changes that were durable over 24 weeks and superior to those seen with tezacaftor plus ivacaftor in this patient population.
Dr Sivagurunathan Sutharsan is in the Department of Pulmonary Medicine, Division of Cystic Fibrosis, University Medicine Essen-Ruhrlandklinik, University of Duisburg-Essen, Essen, Germany.
2022 2022 2022
Lina Merjaneh, Sana Hasan, Nader Kasim, Katie Larson Ode. The role of modulators in cystic fibrosis related diabetes. J Clin Transl Endocrinol 2021 Dec 7;27:100286.doi: 10.1016/j.jcte.2021.100286.eCollection 2022 Mar.Free PMC article [Pubmed]

Lina Merjaneh
The development and introduction of modulator therapies have completely shifted the paradigm for the treatment of cystic fibrosis (CF). Highly effective modulator therapies have driven marked improvements in lung function, exacerbation rate, weight and quality of life in CF patients. However, their effect on CF related diabetes (CFRD) is not well delineated. The role of CF transmembrane conductance regulator (CFTR) in CFRD pathogenesis is inadequately understood and research aimed at deciphering the underlying mechanisms of CFRD continues to evolve.
In this review, we summarize what is known regarding the effect of CFTR modulators on CFRD. Small studies using ivacaftor monotherapy in gating mutations have revealed improvement in insulin secretion, glucose tolerance and/or decrease in insulin requirement. However, lumacaftor/ivacaftor studies (primarily in delta F 508 homozygous) have not revealed significant improvement in CFRD or glucose tolerance. No studies are yet available regarding the effect of the highly effective triple therapy (elexacaftor/tezacaftor/ivacaftor) on CFRD or insulin secretion. CFTR modulators might affect development or progression of CFRD through many mechanisms including improving insulin secretion by correcting the CFTR defect directly, improving ductal function, reducing islet inflammation, and improving incretin secretion or by enhancing insulin sensitivity via reduced systemic inflammation and increased physical activity driven by improved lung function and quality of life. On the other hand, they can stimulate appetite and improve gastrointestinal function resulting in increased caloric intake and absorption, driving excessive weight gain and potentially increased insulin resistance. If the defect in insulin secretion is reversible then it is possible that initiation of CFTR modulators at a younger age might help prevent CFRD. Despite the advances in CF management, CFRD remains a challenge and knowledge continues to evolve. Future studies will drive better understanding of the role of highly effective CFTR modulators in CFRD.
Dr Lina Merjaneh is a pediatric endocrinologist and Assistant Professor at Seattle Children’s Hospital.
Katharina Habler, Anne-Sophie Kalla, Michael Rychlik, Mathias Bruegel, Daniel Teupser, Susanne Nährig, Michael Vogeser, Michael Paal.Isotope dilution LC-MS/MS quantification of the cystic fibrosis transmembrane conductance regulator (CFTR) modulators ivacaftor, lumacaftor, tezacaftor, elexacaftor, and their major metabolites in human serum. Clin Chem Lab Med 2021 Oct 19.doi: 10.1515/cclm-2021-0724. Online ahead of print [Pubmed]

Katharina Habler
Objectives:Cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators have revolutionized the therapeutic landscape in CF treatment. These vital drugs are extensively metabolized via CYP3A, so caution must be exercised in multimodal CF therapy because of the risk of adverse drug interactions. Our goal was to develop a highly sensitive assay for the purpose of therapeutic drug monitoring (TDM) in diagnostic laboratories
Methods:After protein precipitation, the CFTR modulators ivacaftor, lumacaftor, tezacaftor, elexacaftor, and their metabolites ivacaftor-M1, ivacaftor-M6, and tezacaftor-M1 were separated with a two-dimensional chromatography setup within 5 min, and quantified with stable isotope-labeled internal standards. The method was validated according to the European Medicines Agency (EMA) guideline on bioanalytical method validation and applied to CF patient samples.
Results:Inaccuracy was ≤7.0% and the imprecision coefficient of variation (CV) was ≤8.3% for all quality controls (QCs). The method consistently compensated for matrix effects, recovery, and process efficiency were 105-115 and 96.5-103%, respectively. Analysis of CF serum samples provided concentrations comparable to the pharmacokinetic profile data reported in the EMA assessment report for the triple combination therapy Kaftrio.
Conclusions:We hereby present a robust and highly selective isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) assay for the simultaneous quantification of the so far approved CFTR modulators and their metabolites in human serum. The assay is suitable for state-of-the-art pharmacovigilance of CFTR modulator therapy in CF patients, in order to maximize safety and efficacy, and also to establish dose-response relationships in clinical trials.
Katharina Habler is at the Institute of Laboratory Medicine, University Hospital, LMU Munich, Munich, Germany
Hunter Ragan, Elizabeth Autry, Taryn Bomersback, Jennifer Hewlett, Lauren Kormelink, Julie Safirstein, Laura Shanley, Lisa Lubsch. The use of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis post-liver transplant: A case series. Pediatr Pulmonol 2022 Feb;57(2):411-417.doi: 10.1002/ppul.25779.Epub 2021 Dec 12.[Pubmed]
Introduction:Cystic fibrosis (CF)-related liver disease (CFLD) manifests as a wide spectrum of hepatobiliary disease and can progress to need liver transplantation. Elexacaftor/tezacaftor/ivacaftor (elx/tez/iva) is a cystic fibrosis transmembrane conductance regulator modulator that has superior efficacy compared to previously approved modulators. Use of elx/tez/iva, should be approached with caution in individuals with CFLD or following liver transplantation due to possible increases in liver function tests (LFTs) and drug-drug interactions with several immunosuppressant medications.
Objective:The purpose of this case series is to explore if the use of elx/tez/iva is safe and tolerable in patients with CF postliver transplantation.
Methods:A retrospective case series including patients prescribed elx/tez/iva following liver transplantation and an immunosuppressive regimen consisting of drug therapy metabolized by P-glycoprotein was completed.
Results:Ten patients at six CF centers with a median age of 22.1 years (range 14-43.4 years) and the median time from the transplant of 6.9 years (range 0.6-22 years) were included. Most patients (8, 80%) received a reduced or full dose of elx/tez/iva for a mean duration of 10.4 months (range 7-12 months). Fluctuations in LFTs occurred in all patients (10, 100%) and led to therapy discontinuation in two patients (20%). Elx/tez/iva initiation resulted in elevations in tacrolimus trough concentration in seven patients (70%). Most patients who tolerated elx/tez/iva had symptomatic and quality of life improvement, increased body mass index, and maintained or improved lung function.
Conclusion:Initiation of elx/tez/iva in patients with CF who received liver transplantation may be safe with clinical benefits.
Hunter Ragan is at the Goldfarb School of Nursing at Barnes-Jewish College, St. Louis, Missouri, USA.
Renée V. E. Dagenais, Victoria C. Suand Bradley S. Quon Real-World Safety of CFTR Modulators in the Treatment of Cystic Fibrosis: A Systematic Review. J Clin Med.2021 Jan; 10(1): 23. Published online 2020 Dec 23.doi: 10.3390/jcm10010023 [Pubmed]
[This article has been corrected.SeeJ Clin Med. 2022 January 10; 11(2): 318.[Pubmed] Mistake in Table 1. Reference citation [34] was wrong. The corrected Table 1 appears below. The authors state that the scientific conclusions are unaffected. The original article has been updated]

Renee Dagenais
Cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies target the underlying cause of cystic fibrosis (CF), and are generally well-tolerated; however, real-world studies indicate the frequency of discontinuation and adverse events (AEs) may be higher than what was observed in clinical trials. The objectives of this systematic review were to summarize real-world AEs reported for market available CFTR modulators (i.e., ivacaftor (IVA), lumacaftor/ivacaftor (LUM/IVA), tezacaftor/ivacaftor (TEZ/IVA), and elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA)), and to identify ways in which the pharmacist on CF healthcare teams may contribute to mitigating and managing these AEs. The MEDLINE, EMBASE, CINAHL, and Web of Science Core Collection online databases were searched from 2012 to 1 Aug 2020. Full manuscripts or conference abstracts of observational studies, case series, and case reports were eligible for inclusion. The included full manuscripts and conference abstracts comprised of 54 observational studies, 5 case series, and 9 case reports. The types of AEs reported generally aligned with what have been observed in clinical trials. LUM/IVA was associated with a higher frequency of respiratory-related AE and discontinuation in real-world studies. A signal for mental health and neurocognitive AEs was identified with all 4 CFTR modulators. A systematic approach to monitoring for AEs in people with CF on CFTR modulators in the real-world setting is necessary to help better understand potential AEs, as well as patient characteristics that may be associated with higher risk of certain AEs. Pharmacists play a key role in the safe initiation and monitoring of people with CF on CFTR modulator therapies.
Renée V. E. Dagenais from the Adult Cystic Fibrosis Program, St. Paul’s Hospital, Vancouver, BC V6Z 1Y6, Canada. Department of Pharmacy, St. Paul’s Hospital, Vancouver, BC V6Z 1Y6, Canada and the Centre for Heart Lung Innovation, St. Paul’s Hospital, Vancouver, BC V6Z 1Y6, Canada.
– This is a useful encyclopaedic article on the downside of the new modulator drugs
Thomas S FitzMaurice, Caroline McCann, Dilip Nazareth, Matthew Shaw, Paul S McNamara, Martin J Walshaw. Measuring the effect of elexacaftor/tezacaftor/ivacaftor combination therapy on the respiratory pump in people with CF using dynamic chest radiography. J Cyst Fibros 2022 Jan 28;S1569-1993(22)00027-3. doi: 10.1016/j.jcf.2022.01.007.Online ahead of print. [Pubmed]

Thomas S FitzMaurice
Background: The CFTR modulator elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) leads to significant improvement in the symptoms and spirometry of people with cystic fibrosis (pwCF), but little evidence exists to understand its effect on respiratory pump function. Dynamic chest radiography (DCR) is a novel cineradiographic tool that identifies and tracks the chest wall and diaphragm throughout the breathing cycle, alongside fluoroscopic images of the chest of diagnostic quality.
Methods: In this observational work, we examined the spirometry and DCR of 24 pwCF before and after starting ELX/TEZ/IVA. DCR automatically tracked the hemidiaphragm midpoints and projected lung area (PLA) during tidal and deep breathing manoeuvres.
Results: ppFEV1 (61±18 to 73±22, P<0.001) and ppFVC (77±16 to 88±15, P<0.001) improved significantly. DCR demonstrated a significant increase in hemidiaphragm excursion on both the right (18±11 to 26±9 mm, P<0.001) and left (21±11 to 31±11 mm, P<0.001) sides, as well as maximum hemidiaphragm speed during inspiration (right 22±14 to 31±11 mm/s, P=0.03; left 28±11 to 37±16 mm/s, P=0.02). PLA at end-expiration was significantly reduced (334±71 to 290±72cm2, P<0.001), with a significant increase in ΔPLA (83±40 to 117±36cm2, P<0.001).
Conclusions: DCR demonstrated significant improvements in hemidiaphragm excursion and ΔPLA in pwCF started on ELX/TEZ/IVA. These changes likely reflect a reduction in air trapping and improved elastic recoil of the chest, and are consistent with improvements seen in spirometry. The changes seen with DCR are physiologically plausible and correlate well with spirometry. DCR warrants further investigation as a tool for assessing the impact of CFTR-modulating therapies.
Dr Thomas S FitzMaurice is a Fellow in the Adult CF Unit, Liverpool Heart and Chest Hospital, Thomas Drive, Liverpool L14 3PE, UK; Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK.
B L Aalbers, J E Brunsveld, C K van der Ent, J C van den Eijnden, J M Beekman, H G M Heijerman. Forskolin induced swelling (FIS) assay in intestinal organoids to guide eligibility for compassionate use treatment in a CF patient with a rare genotype. J Cyst Fibros 2022 Jan 30;S1569-1993(22)00028-5.doi: 10.1016/j.jcf.2022.01.008. Online ahead of print. [Pubmed]
Background: Forskolin-induced swelling of patient-derived organoids has been used to measure patient-specific CFTR function and CFTR modulator response. We present a case where CFTR function assessment in intestinal organoids was decisive for a patients’ acceptance to a compassionate use program.
Case description: A 56 years old female with cystic fibrosis compound heterozygous for F508del and a rare CFTR allele (c.3717+5G>T) experienced rapid clinical deterioration. The forskolin-induced swelling assay on her rectal organoids was used to confirm that the rare mutation is a minimal residual function mutation, and that other CFTR modulators would not likely be effective. Based on these two criteria and her clinical status, she was accepted for compassionate use of elexacaftor/tezacaftor/ivacaftor and showed improvement in all clinical parameters.
Conclusions: This reports describes a first example that intestinal organoids were used to identify a previously unknown CFTR mutation as a minimal function mutation. The individual FIS-based definition of minimal residual function, response to ele/tez/iva and/or lack of response to other CFTR modulating drugs, may thus provide a tool for access to ele/tez/ivatreatment for people with rare genotypes.
B L Aalbers is in the Department of pulmonology, UMC Utrecht, The Netherlands. Electronic address: b.l.aalbers-2@umcutrecht.nl.
Danya Muilwijk, Eyleen de Poel, Peter van Mourik, Sylvia W F Suen, Annelotte M Vonk , Jesse E Brunsveld et al.(please see PubMed abstract for full author list). Forskolin-induced Organoid Swelling is Associated with Long-term CF Disease Progression. Eur Respir J 2022 Jan 27;2100508.doi: 10.1183/13993003.00508-2021. Online ahead of print. [Pubmed]

Danya Muilwijk
Rationale: Cystic fibrosis (CF) is a monogenic life-shortening disease associated with highly variable individual disease progression which is difficult to predict. Here we assessed the association of forskolin-induced swelling (FIS) of patient-derived organoids (PDO) with long-term CF disease progression in multiple organs and compared FIS with the golden standard biomarker sweat chloride concentration (SCC).
Methods: We retrieved 9-year longitudinal clinical data from the Dutch CF Registry of 173 people with mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Individual CFTR function was defined by FIS, measured as the relative size increase of intestinal organoids after stimulation with 0.8 µM forskolin, quantified as area under the curve (AUC). We used linear mixed effect models and multivariable logistic regression to estimate the association of FIS with long-term FEV1pp decline and development of pancreatic insufficiency, CF-related liver disease and diabetes. Within these models, FIS was compared with SCC.
Results: FIS was strongly associated with longitudinal changes of lung function, with an estimated difference in annual FEV1pp decline of 0.32% (95%CI: 0.11%-0.54%; p=0.004) per 1000-points change in AUC. Moreover, increasing FIS levels were associated with lower odds of developing pancreatic insufficiency (adjusted OR: 0.18, 95%CI: 0.07-0.46, p<0.001), CF-related liver disease (adjusted OR: 0.18, 95%CI: 0.06-0.54, p=0.002) and diabetes (adjusted OR: 0.34, 95%CI: 0.12-0.97, p=0.044). These associations were absent for SCC.
Conclusion: This study exemplifies the prognostic value of a PDO-based biomarker within a clinical setting, which is especially important for people carrying rare CFTR mutations with unclear clinical consequences.
Dr Danya Muilwijk is a PhD candidate in the Department of Pediatric Respiratory Medicine, Wilhelmina Children’s Hospital, University Medical Center, Utrecht University, Utrecht, The Netherlands.
Eyleen de Poel is in the Department of Regenerative Medicine Utrecht, University Medical Center, Utrecht University, Utrecht, The Netherlands.
Aaron C Miller, Logan M Harris , Joseph E Cavanaugh, Mahmoud Abou Alaiwa, David A Stoltz, Douglas B Hornick, Philip M Polgreen. The rapid reduction of infection-related visits and antibiotic use among people with cystic fibrosis after starting Elexacaftor-Tezacaftor-Ivacaftor. Clin Infect Dis 2022 Feb 10;ciac117.doi: 10.1093/cid/ciac117. Online ahead of print. [Pubmed

Aaron Miller
Background: People with cystic fibrosis (CF) routinely suffer from recurrent sino-pulmonary infections. Such infections require frequent courses of antimicrobials and often involve multidrug-resistant organisms. The goal of this study was to identify real-world evidence for the effectiveness of Elexacaftor-Tezacaftor-Ivacaftor (ELX/TEZ/IVA) at decreasing infection-related visits and antimicrobial use in people with CF.
Methods: Using IBM MarketScan data, we identified 389 enrollees with CF who began taking ELX/TEZ/IVA prior to 12/1/2019 and were enrolled from 7/1/2019-3/14/2020. We also identified a comparison population who did not begin ELX/TEZ/IVA during the study period. We compared the following outcomes in the 15 weeks before and after medication initiation: total healthcare visits, inpatient visits, infection-related visits, and antimicrobial prescriptions. We analyzed outcomes using both a case-crossover analysis and a difference-in-differences analysis, to control for underlying trends.
Results: For the case-crossover analysis, ELX/TEZ/IVA initiation was associated with 2.20 (95% CI: -3.26, -1.14) fewer overall healthcare visit-days, 0.16 (95% CI: -0.22, -0.11) fewer inpatient admissions, 0.33 (95% CI: -0.59, -0.07) fewer infection-related visit-days, and 0.78 (95% CI: -1.03, -0.54) fewer antibiotic prescriptions over a 15 week period. Results from the difference-in-differences approach were similar.
Conclusions: We show a rapid reduction of infection-related visits and antimicrobial use among people with CF after starting a therapy that was not explicitly designed to treat infections. Currently, there are over 30,000 people living with CF in the United States alone. Given that this therapy is effective for approximately 90% of people with CF, the impact on respiratory infections and antimicrobial use may be substantial.
Dr Aaron C Miller is at the Department of Internal Medicine, University of Iowa, Iowa City, IA, USA.
Gregory S Sawicki, Mark Chilvers, John McNamara, Lutz Naehrlich, Clare Saunders, Isabelle Sermet-Gaudelus, Claire E Wainwright, Neil Ahluwalia, Daniel Campbell, R Scott Harris, Hildegarde Paz-Diaz, Judy L Shih, Jane C Davies. A Phase 3, open-label, 96-week trial to study the safety, tolerability, and efficacy of tezacaftor/ivacaftor in children ≥ 6 years of age homozygous for F508del or heterozygous for F508del and a residual function CFTR variant. J Cyst Fibros 2022 Feb 18;S1569-1993(22)00033-9.doi: 10.1016/j.jcf.2022.02.003.Online ahead of print.[Pubmed]

Gregory Sawicki
Background: Two previous Phase 3 studies (“parent studies”) showed that tezacaftor/ivacaftor was generally safe and efficacious for up to 24 weeks in children 6 through 11 years of age with cystic fibrosis (CF) and F508del/F508del (F/F) or F508del/residual function (F/RF) genotypes. We assessed the safety and efficacy of tezacaftor/ivacaftor in an open-label, 96-week extension study.
Methods: This was a Phase 3, 2-part, multicenter, open-label, extension study in children 6 through 11 years of age at treatment initiation (Study VX17-661-116; NCT03537651). The primary endpoint was safety and tolerability. Secondary endpoints were absolute change from baseline in lung clearance index2.5 (LCI2.5), sweat chloride (SwCl) concentration, Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score, and body mass index (BMI).
Results: One-hundred thirty children enrolled and received ≥ 1 dose of tezacaftor/ivacaftor; 109 completed treatment. Most (n = 129) had ≥ 1 treatment-emergent adverse event (TEAE), the majority of which were mild or moderate in severity and generally consistent with common manifestations of CF. Exposure-adjusted TEAE rates were similar to or lower than those in the parent studies. Five (3.8%) had TEAEs leading to treatment discontinuation. Efficacy results from the parent studies were maintained, with improvements in lung function, SwCl concentration, CFQR respiratory domain score, and BMI observed from parent study baseline to Week 96.
Conclusions: Tezacaftor/ivacaftor is generally safe and well tolerated, and treatment effects are maintained for up to 120 weeks. These results support long-term use of tezacaftor/ivacaftor in children ≥ 6 years of age with CF and F/F or F/RF genotypes.
Gregory S Sawicki is at the Boston Children’s Hospital, Harvard Medical School, 300 Longwood Avenue, Boston, MA,
Dirk Westhölter FabianSchumacher, Nuria Wülfinghoff, SivagurunathanSutharsan, Svenja Strassburg, Burkhard Kleuser, Peter A Horn, Sebastian Reuter, Erich Gulbins, Christian Taube, Matthias Welsner. CFTR modulator therapy alters plasma sphingolipid profiles in people with cystic fibrosis. J Cyst Fibros 2022 Feb 12;S1569-1993(22)00037-6.doi: 10.1016/j.jcf.2022.02.005.Online ahead of print. [Pubmed]

Dirk Westholter
Background: Sphingolipids, in particular ceramides, play an important role in the pathogenesis of cystic fibrosis (CF) lung disease. Ceramides seem to be dysregulated in people with CF (PWCF): An elevated ratio of ceramides C16Cer/ C24Cer has been linked to inflammation and disease severity. CFTR modulators might influence sphingolipid dysregulation in PWCF.
Methods: Sphingolipid profiles were retrospectively analyzed in serum from 112 PWCF and 96 healthy controls as well as in plasma from 25 PWCF before and after treatment with the CFTR modulator elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Lipid data were correlated with clinical parameters.
Results: There were significantly higher levels of long-chain ceramides C18Cer and C20Cer and of the very long-chain ceramide C24:1Cer in PWCF versus healthy controls. Sphingosine levels were significantly reduced and accurately distinguished PWCF from healthy controls. Treatment with ELX/TEZ/IVA was associated with a decrease in levels of long-chain ceramides C16Cer, C18Cer and C20Cer and very long-chain ceramide C24:1Cer. Plasma levels of the most abundant very long-chain ceramide C24Cer as well as sphingosine-1-phosphate increased. Consequently, the ratio of ceramides C16Cer/ C24Cer decreased. Sphingolipid levels showed weak correlations with clinical parameters.
Conclusions: These findings highlight the existence of a distinctive sphingolipid profile in blood from PWCF, which appears to be altered by ELX/TEZ/IVA therapy. Thus, strategies for sphingolipid remodeling need to be reassessed and adjusted in the light of highly effective CFTR modulator therapies.
Dirk Westhölter is in the Department of Pulmonary Medicine, University Hospital Essen- Ruhrlandklinik, Tüschener Weg 40, Essen 45239, Germany. Electronic address: dirk.westhoelter@uk-essen.de.
Teresa Fuchs, Dorothea Appelt, Katharina Niedermayr, Helmut Ellemunter. REAL-world clinical effectiveness of ivacaftor therapy in the first 24 months in two infants with cystic fibrosis and different gating mutations-A case report. Clin Case Rep 2022 Feb 10;10(2):e05364.doi: 10.1002/ccr3.5364.eCollection 2022 Feb Free PMC article[Pubmed]
This study summarizes efficacy of ivacaftor treatment in 2 infants in a real-world setting. Two infants aged 2 and 11 months were started on ivacaftor off label with parental consent. A distinct decline of sweat chloride and lung clearance index plus increase in fecal elastase was seen. No side effects occurred. The results underline the early and sustainable effect in patients started aged less than 12 months and give cause for discussing whether a reduction in standard cystic fibrosis therapy is possible. Further details in the Free PMC article where other studies on young infants are discussed.
Teresa Fuchs is at the Medical University of Innsbruck Cystic Fibrosis Centre Innsbruck Innsbruck Austria
Ann Cheng, Olivia Baker, Uta Hill. Elexacaftor, tezacaftor and ivacaftor: a case of severe rash and approach to desensitisation. BMJ Case Rep 2022 Mar 2;15(3):e247042. doi: 10.1136/bcr-2021-247042. [Pubmed]

Ann Cheng
We present a case of severe rash following induction of elexacaftor, tezacaftor and ivacaftor (ELX/TEZ/IVA) in a young adult male cystic fibrosis patient. While rash is a commonly reported side effect which resolves in 1-2 weeks with minimal intervention, our patient had presented with fever and widespread rash prompting medication cessation. After a washout period, reintroduction with 1/2 tablet of ELX/TEZ/IVA produced a similar systemic response within 24 hours. Repeat attempt, this time with 1/8 tablet and increasing in increments of an eighth daily, was successful and has allowed our patient to experience the transformative benefits of ELX/TEZ/IVA including improved pulmonary function and reduced episodes of infective exacerbation. This case illustrates one of the most common side effects of ELX/TEZ/IVA triple therapy, and our experience of desensitisation to ELX/TEZ/IVA in a challenging case of rash.
Dr Ann Cheng is NIHR Academic Clinical Fellow in the Department of Infection, Immunity and Cardiovascular Disease, The University of Sheffield, Sheffield, UK. Also Cambridge Centre for Lung Infection, Royal Papworth Hospital, Cambridge, UK.
Julie Mésinèle , Manon Ruffin, Loïc Guillot, Pierre-Yves Boëlle, Harriet Corvol, On Behalf Of The French Cf Modifier Gene Study Investigators. Factors predisposing the response to Lumacaftor/ivacaftor in people with cystic fibrosis. J Pers Med 2022 Feb 10;12(2):252.doi: 10.3390/jpm12020252.[Pubmed]Free PMC article
Lumacaftor/ivacaftor (LUMA-IVA) therapy is prescribed to people with cystic fibrosis (pwCF) homozygous for the Phe508del-CFTR variant to restore CFTR protein function. There is, however, large inter-individual variability in treatment response. Here, we seek to identify clinical and/or genetic factors that may modulate the response to this CFTR modulator therapy.
A total of 765 pwCF older than 12 years under LUMA-IVA therapy and with lung function and nutritional measurements available before and after treatment initiation were included. Response to treatment was determined by the change in lung function and nutritional status, from baseline and over the first two years after initiation, and it was assessed by weighted generalized estimating equation models. Gains in lung function and nutritional status were observed after 6 months of treatment (on average 2.11 ± 7.81% for percent predicted FEV1 and 0.44 ± 0.77 kg/m2 for BMI) and sustained over the 2 years.
We observed that the more severe patients gained the most in lung function and nutritional status. While females started with a nutritional status more impaired than males, they had a larger response and regained BMI Z-score values similar to men after 2 years of treatment. We observed no association between variants in solute carrier (SLC) genes and the respiratory function response to LUMA-IVA, but the SLC6A14 rs12839137 variant was associated with the nutritional response. Further investigations, including other genomic regions, will be needed to fully explore the inter-individual variability of the response to LUMA-IVA.
Dr Julie Mésinèle is Post-Doctoral Researcher in Biostatistics at the Centre de Recherche Saint-Antoine (CRSA), Inserm, Sorbonne Université, 75012 Paris, France. and Hôpital Saint-Antoine, AP-HP, Institut Pierre Louis d’Epidémiologie et de Santé Publique (iPLESP), Inserm, Sorbonne Université, 75012 Paris, France
Samantha L Durfey, Sudhakar Pipavath, Anna Li, Anh T Vo, Anina Ratjen, Suzanne Carter, Sarah J Morgan, Matthew C Radey, Brenda Grogan, Stephen J Salipante, Michael J Welsh, David A Stoltz, Christopher H Goss , Edward F McKone , Pradeep K Singh./ Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections. mBio 2021 Dec 21;12(6):e0314821. doi: 10.1128/mbio.03148-21.Epub 2021 Dec 14. Free PMC article [Pubmed]

Samantha Durfey
Drugs called CFTR modulators improve the physiologic defect underlying cystic fibrosis (CF) and alleviate many disease manifestations. However, studies to date indicate that chronic lung infections that are responsible for most disease-related mortality generally persist.
Here, we investigated whether combining the CFTR modulator ivacaftor with an intensive 3.5-month antibiotic course could clear chronic Pseudomonas aeruginosa or Staphylococcus aureus lung infections in subjects with R117H-CFTR, who are highly ivacaftor-responsive. Ivacaftor alone improved CFTR activity, and lung function and inflammation within 48 h, and reduced P. aeruginosa and S. aureus pathogen density by ∼10-fold within a week. Antibiotics produced an additional ∼10-fold reduction in pathogen density, but this reduction was transient in subjects who remained infected. Only 1/5 P. aeruginosa-infected and 1/7 S. aureus-infected subjects became persistently culture-negative after the combined treatment. Subjects appearing to clear infection did not have particularly favourable baseline lung function or inflammation, pathogen density or antibiotic susceptibility, or bronchiectasis scores on CT scans, but they did have remarkably low sweat chloride values before and after ivacaftor. All persistently P. aeruginosa-positive subjects remained infected by their pre-treatment strain, whereas subjects persistently S. aureus-positive frequently lost and gained strains. This work suggests chronic CF infections may resist eradication despite marked and rapid modulator-induced improvements in lung infection and inflammation parameters and aggressive antibiotic treatment.
IMPORTANCE Recent work shows that people with CF and chronic lung infections generally remain persistently infected after treatment with drugs that target the CF physiological defect (called CFTR modulators). However, changes produced by modulators could increase antibiotic efficacy. We tested the approach of combining modulators and intensive antibiotics in rapid succession and found that while few subjects cleared their infections, combined treatment appeared most effective in subjects with the highest CFTR activity. These findings highlight challenges that remain to improve the health of people with CF.
Dr Samantha L Durfey is in the Department of Microbiology, University of Washington School of Medicinegrid.471394.c, Seattle, Washington, USA.
Lauryn A Benninger, Cesar Trillo, Jorge Lascano. CFTR modulator use in post lung transplant recipients. J Heart Lung Transplant 2021 Dec;40(12):1498-1501.doi: 10.1016/j.healun.2021.08.009.Epub 2021 Aug 26. [Pubmed]

Lauryn Benninger
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator therapy has previously been contraindicated in solid organ transplant recipients. This was due to lack of data and concern for interactions with immunosuppressive drug regimens. However, in post-lung transplant recipients, CFTR modulators may improve extrapulmonary manifestations of cystic fibrosis without impacting graft function or immunosuppressive drug levels. Herein, we present our single center experience with the use of elexacaftor/tezacaftor/ivacaftor, Trikafta, in adult post-lung transplant recipients.
Dr Lauryn A Benninger is a pulmonologist in the Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Florida College of Medicine, Gainesville, Florida
Clémence Martin, Martine Reynaud-Gaubert, Rebecca Hamidfar, Isabelle Durieu, Marlène Murris-Espin, Isabelle Danner-Boucher, Raphaël Chiron, Sylvie Leroy, Benoit Douvry, Dominique Grenet. Laurent Mely, Sophie Ramel, Sylvie Montcouquiol, LydieLemonnier, Espérie Burnet, Jean-Louis Paillasseur, Jennifer Da Silva, Pierre-Régis Burgel. Sustained effectiveness of elexacaftor-tezacaftor-ivacaftor in lung transplant candidates with cystic fibrosis. J Cyst Fibros. 2022 Feb 2;S1569-1993(22)00032-7.doi: 10.1016/j.jcf.2022.01.012.Online ahead of print [Pubmed]

Clemence Martin
Background: Elexacaftor-tezacaftor-ivacaftor induces rapid clinical improvement in patients with cystic fibrosis (CF) and advanced pulmonary disease, often leading to suspend the indication for lung transplantation. Yet no long-term data is available in lung transplant candidates.
Methods: Lung transplant candidates (defined as being waitlisted for lung transplantation or considered for listing within 3 months) who have initiated elexacaftor-tezacaftor-ivacaftor were identified in the French cohort of patients with CF and advanced pulmonary disease. Patients were prospectively followed to evaluate treatment safety and effectiveness from initiation to July 20th, 2021.
Results: Among the 331 patients with advanced CF pulmonary disease who initiated elexacaftor-tezacaftor-ivacaftor, 65 were lung transplant candidates (17 listed for transplantation, 48 considered for listing within 3 months). Median [IQR] follow-up time was 363 [329; 377] days. At the end of the follow-up period, two patients were transplanted five and 11 days following treatment initiation, two were listed for transplantation, and 61 no longer met transplantation criteria. Improvement in percent predicted forced expiratory volume in 1 s (ppFEV1) at one month was +13.4% (95% confidence interval, 10.3%-16.5%; P < 0.0001) and remained stable thereafter. Treatment burden decreased substantially, with an 86% decrease in the need for intravenous antibiotics, 59% for oxygen therapy and 62% for non-invasive ventilation.
Conclusion: In lung transplant candidates eligible for elexacaftor-tezacaftor-ivacaftor, the rapid improvement following initiation of treatment persisted over one year with a reduction in treatment burden and lung transplantation could be safely deferred in most patients.
Clémence Martin is at the Université de Paris, Institut Cochin, InsermU1016, Paris, France; Respiratory Medicine and Cystic Fibrosis National Reference Center, Cochin Hospital, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France; ERN-Lung CF network, Frankfurt, Germany.
Ciaran A Shaughnessy, Pamela L Zeitlin, Preston E Bratcher. Net benefit of ivacaftor during prolonged tezacaftor/elexacaftor exposure in vitro. J Cyst Fibros 2022 Mar 2;S1569-1993(22)00043-1.doi: 10.1016/j.jcf.2022.02.011.Online ahead of print. [Pubmed]

Ciaran A Shaughnessy
Background: A decrease in the lumacaftor-mediated increase in F508del-CFTR function and expression upon prolonged exposure to ivacaftor (VX-770) has previously been described. However, the efficacy observed with ivacaftor-containing CFTR modulator therapies in vivo is in conflict with these reports. We hypothesized that a portion of the apparent decrease in CFTR function observed after prolonged ivacaftor exposure in vitro was due to an increase in constitutive CFTR-mediated ion transport.
Methods: Human nasal epithelial (HNE) cells were obtained by brushings from three CF individuals homozygous for the F508del CFTR mutation. Differentiated epithelia were pre-treated with prolonged (24 h) exposure to either lumacaftor (VX-809; 3 µM), tezacaftor (VX-661; 3 µM), elexacaftor (VX-445; 3 µM), and/or ivacaftor (0.1-6.4 µM) or DMSO (vehicle control), and CFTR function was assayed by Ussing chamber electrophysiology.
Results: In cells treated with lumacaftor, constitutive CFTR activity was not increased at any concentration of co-treatment with ivacaftor. Constitutive CFTR activity was also unchanged in cells treated with the combination of tezacaftor and elexacaftor. An increase in constitutive CFTR activity above the DMSO controls was only observed in cells treated with the combination of tezacaftor and elexacaftor and co-treated with at least 0.1 µM ivacaftor.
Conclusions: These results demonstrate that ivacaftor is a critical component in the triple combination therapy along with tezacaftor and elexacaftor to increase constitutive CFTR function. This work further elucidates the mechanism of action of the effective triple combination therapeutic that is now the primary clinical tool in treating CF.
Ciaran A Shaughnessy is National Science Foundation Postdoctoral Fellow in the Department of Pediatrics, National Jewish Health, Denver, CO.
Amanda L Stapleton, Adam J Kimple, Jennifer L Goralski, S Mehdi Nouraie, Barton F Branstetter, Amber D Shaffer, Joseph M Pilewski, Brent A Senior, Stella E Lee, Anna C Zemke. Elexacaftor-Tezacaftor- Ivacaftor improves sinonasal outcomes in cystic fibrosis. J Cyst Fibros 2022 Mar 14;S1569-1993(22)00051-0.doi: 10.1016/j.jcf.2022.03.002.Online ahead of print. Free article [Pubmed]

Amanda Stapleton
Background: Many individuals with cystic fibrosis (CF) have chronic rhinosinusitis resulting in nasal obstruction, sinus infections, and repeated surgeries. Elexacaftor-tezacaftor-ivacaftor is a highly effective modulator therapy approved for individuals aged 6 years or older with CF who have at least one F508del allele or other responsive mutation. The current study tests the hypothesis that ELX/TEZ/IVA improves sinonasal disease in CF.
Methods: The study was a pre/post, observational cohort study conducted at two sites. Participants underwent a study visit prior to starting ELX/TEZ/IVA and a second visit at a median of 9 months on therapy. Each visit included sinus CT scan, rigid nasal endoscopy, and sweat chloride measurement. Symptoms were measured with the 22 item Sinonasal Outcome Test at scheduled intervals during the study. Regression models were used to test for improvement in symptoms, endoscopy, and CT scales.
Results: The study enrolled 34 individuals, with a median age of 27 years (range 12-60). Symptoms improved within 7 days of therapy and plateaued by day 28. Endoscopic crusting resolved and nasal polyposis improved, with a decrease in size or resolution of polyps. Sinus opacification and mucosal thickening improved on CT radiographs with treatment.
Conclusions: Sinonasal symptoms improved rapidly and durably for at least 180 days on ELX/TEZ/IVA therapy. Objective measures of disease including endoscopic and CT findings improved with ELX/TEZ/IVA.
Dr Amanda L Stapleton is Associate Professor in the Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh, United States.
Marco Zampoli, Nataliya Kashirskaya, Bulent Karadag, Luiz Vicente Ribeiro Ferreira da Silva Filho, Grace R Paul,Christine Noke. Global access to affordable CFTR modulator drugs: Time for action! J Cyst Fibros 2022 Mar 24;S1569-1993(22)00083-2.doi: 10.1016/j.jcf.2022.03.006.Online ahead of print.

Marco Zampoli
There is no abstract so the article is reproduced here
Whilst the incredible advancements in CF treatment are to be applauded and celebrated, the reality is that only a minority of people with CF in the rich global North are benefiting from CFTRm therapy. We are therefore very grateful and encouraged by the timely and important article by Jonathan Guo et al. published recently in the Journal which highlights the stark reality and disparity that exists in CF diagnosis and treatment across the world. According to their estimates based on available data, there are 57,076 people with undiagnosed CF across the world with the highest burden likely to be in the Middle East and India. Furthermore, only 12% of estimated 105,352 people captured in global CF registries are receiving Trikafta®/Kaftrio® and the majority of them are living in North America or Western Europe. We agree with their view that paucity of epidemiological data and lack of CF diagnosis capacity in low and middle-income countries (LMICs) are major barriers to advocating for effective and equitable treatment in these regions. However, there are significant CF populations living in LMICs such as South Africa, India, the Middle East, eastern Europe, and South America who currently have no access nor pathway to registration of Trikafta®/Kaftrio® which will undoubtably lead to increasing disparity in CF outcomes between the rich global North and poor global South. In these countries, the main barrier to accessing triple CFTRm therapy is not lack of diagnosis capacity, but profit-driven global market forces which are accountable more to shareholders than the health needs of the global CF community.
Global distribution and sales of CFTRm drugs and patents are in place until 2037 in many LMICs that are signatories to the World Trade Organization Agreement on Trade-Related Aspects of Intellectual Property Rights, effectively preventing manufacture and distribution of affordable generic alternatives in these countries. The current annual cost per patient of Trikafta®/Kaftrio® paid by countries with negotiated agreements is more than $250 000 which is prohibitive for governments and private health insurers in LMICs. Vertex Pharmaceuticals posted a net income of $5.7 billion in 2021 which is a bitter pill to swallow for CF communities in LMICs who are helpless and increasingly impatient with the injustice of their plight . Precedent exists where pharmaceutical companies holding patents have issued voluntary licenses to generic manufacturers to distribute, with profit, life-saving affordable generic drugs in LMICs for treatment of Human Immunodeficiency Virus, hepatitis-C and more recently COVID-19. It is time that the global CF community stand in solidarity in support of similar issuing of voluntary licenses for the manufacture and distribution of generic CFTRm drugs.
We appeal and urge that the Journal and CF patient organizations in the global North such as the Cystic Fibrosis Foundation and European CF Society adopt a stronger position and publish statements against the injustice of the disparity in access to CFTRm therapy for all people across the world living with CF who are eligible for this life-saving treatment. Cystic fibrosis clinicians, researchers and CF communities living in LMICs cannot endure and tolerate much longer reading and hearing about the ‘miracle’ outcomes of triple CFTRm drugs of the privileged minority in rich countries. In the meantime, we continue to patiently negotiate in good faith with Vertex Pharmaceuticals in the hope that CF communities in LMICs will soon have affordable access to this life saving treatment. But time is running out for many people slowly dying too early with CF.
Dr Marco Zampoli is Clinical Head: Cystic Fibrosis Clinic Red Cross War Memorial Children’s Hospital, National Director South African CF Registry Steering Committee, Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Petersen MC, Begnel L, Wallendorf M, Litvin M. Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. Journal of Cystic Fibrosis. 21(2):265-271, 2022 03. [Pubmed]

Max Petersen
Background: Though weight gain has been reported in some clinical trials of CFTR modulators, the effect of elexacaftor-tezacaftor-ivacaftor on body weight, body mass index (BMI), blood pressure, lipids and glycemic control in the real-world setting remains incompletely described.
Methods: We performed a single-center, retrospective, observational analysis of the effect of elexacaftor-tezacaftor-ivacaftor on body weight and cardiometabolic parameters in 134 adult CF patients of the Washington University Adult Cystic Fibrosis Center. Body weight, BMI, and blood pressure were extracted from outpatient clinic visits for the year preceding and the period following the initiation of elexacaftor-tezacaftor-ivacaftor. Other metabolic parameters were extracted at baseline and at latest available follow-up
Results: A mean of 12.2 months of follow-up data was available for analysis. The mean rate of change in BMI was 1.47 kg/m2/yr (95% CI, 1.08 to 1.87) greater after initiation of elexacaftor-tezacaftor-ivacaftor. Significant increases in blood pressure were observed. In those without CFRD, random blood glucose and hemoglobin A1c were decreased after elexacaftor-tezacaftor-ivacaftor initiation. In those with CFRD, elexacaftor-tezacaftor-ivacaftor increased serum total cholesterol, HDL-cholesterol, and LDL-cholesterol.
Conclusions: In this single-center, retrospective, observational study of 134 adults with CF, initiation of elexacaftor-tezacaftor-ivacaftor was associated with increases in BMI at a mean follow up of 12.2 months. Changes in other cardiometabolic risk factors were also observed. Widespread use of elexacaftor-tezacaftor-ivacaftor may be expected to increase the incidence of overnutrition in the CF population.
Max C Petersen is in the Division of Endocrinology, Metabolism, & Lipid Research, Department of Medicine, Washington University, St. Louis, Missouri, USA.
Hannah Farley, Sarah Poole, Stephen Chapman, William Flight. Diagnosis of cystic fibrosis in adulthood and eligibility for novel CFTR modulator therapy. Postgrad Med J 2022 May;98(1159):341-345.doi: 10.1136/postgradmedj-2020 [Pubmed]

Hannah Farley
Background: Cystic fibrosis (CF) is an autosomal recessive condition that primarily manifests as a chronic respiratory disease. CF is usually diagnosed in early childhood or through newborn screening although in a small but important group, diagnosis is not made until adulthood. Highly effective cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies are now available for most genetic causes of CF highlighting the importance of identifying people with late presentations of CF.
Aim: We aimed to identify the clinical characteristics of people diagnosed with CF in adulthood and their resulting eligibility for novel CFTR modulator therapies.
Methods: Patients diagnosed with CF at age 18 years or older were identified from a patient database. Paper and electronic medical records were reviewed and clinical, microbiological and radiological data at diagnosis were recorded.
Results: Nineteen patients were identified. Median age at diagnosis was 38 years (range: 19-71) and 9 (47%) were female. All patients had a history of chronic respiratory symptoms and 18/19 (94%) had radiological evidence of bronchiectasis. All patients had two pathogenic CFTR mutations identified with 16/19 (84%) compound heterozygotes for the F508del mutation. The majority of patients had a CFTR genotype considered eligible for CFTR modulator therapy (84% and 89% according to European and US licences, respectively).
Conclusions: Adult patients with unexplained chronic bronchiectasis should be thoroughly investigated for CF. A low index of suspicion will help to identify adults with undiagnosed CF who are likely to benefit from CFTR modulator therapy.
Dr Hannah Farley is at the Medical School, Oxford University, Oxford, UK
Kathleen J Ramos, Jennifer S Guimbellot, Maryam Valapour, Lauren E Bartlett, Travis Hee Wai, Christopher H Goss, Joseph M Pilewski, Albert Faro, Joshua M Diamond, CFLTC Study Group. Use of elexacaftor/tezacaftor/ivacaftor among cystic fibrosis lung transplant recipients. J Cyst Fibros 2022 Apr 23;S1569-1993(22)00098-4.doi: 10.1016/j.jcf.2022.04.009.Online ahead of print.[Pubmed]

Kathleen J Ramos
Background:Cystic fibrosis (CF) lung transplant (LT) recipients may warrant treatment with elexacaftor/tezacaftor/ivacaftor (ETI) to improve extrapulmonary manifestations of CF. Our objectives were to identify reasons for prescribing ETI after LT and evaluate changes in body mass index (BMI), hemoglobin A1c, hemoglobin, and liver enzymes.
Methods:This was an electronic health record-based cohort study, October 2019-September 2020, at 14 CF LT Consortium sites in North America. The study included CF LT recipients prescribed ETI after transplant. Differences in BMI, A1c, and hemoglobin were assessed with paired t-tests.
Results:There were 94 patients prescribed ETI; indications included sinus disease (68%), GI symptoms (39%), or low BMI (19%). Prescriptions were written by CF physicians (34%), LT physicians (27%), or physicians who practice both CF and LT (39%). Forty patients (42%) stopped ETI at a median of 56 days [IQR 26, 139] after start/prescription date. ETI was not associated with a significant change in BMI (0.2 kg/m2, 95% CI [-0.1, 0.6], p = 0.150), but was associated with decreased A1c (0.4%, 95% CI 0.2, 0.7, p = 0.003), and increased hemoglobin for patients with anemia (0.6 g/dL, 95% CI 0.2, 1.0, p = 0.007). Three people (3%) stopped ETI due to elevated transaminases.
Conclusions:ETI is rarely prescribed for non-pulmonary indications after LT for CF. Further study is needed to determine the risks and benefits of ETI in the CF lung transplant population given the potential for drug interactions, side effects leading to discontinuation of ETI, and the possible mechanisms for ETI to positively impact long-term post-transplant outcomes.
Kathleen J Ramos is in the Department of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington, 1959 NE Pacific Street, Box 356522, Seattle, WA 98195, USA.
David P Nichols, Alex C Paynter, Sonya L Heltshe, Scott H Donaldson, Carla A Frederick, Steven D Freedman and the other members of the PROMISE Study group. Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial. Am J Respir Crit Care Med. 2022 Mar 1;205(5):529-539 doi: 10.1164/rccm.202108-1986OC Free PMC article [Pubmed]

David Nichols
Rationale: The cystic fibrosis (CF) modulator drug, elexacaftor/tezacaftor/ivacaftor (ETI), proved highly effective in controlled clinical trials for individuals with at least one F508del allele, which occurs in at least 85% of people with CF.
Objectives: PROMISE is a post-approval study to understand the broad effects of ETI through 30 months’ clinical use in a more diverse U.S. patient population with planned analyses after 6 months.
Methods: Prospective, observational study in 487 people with CF age 12 years or older with at least one F508del allele starting ETI for the first time. Assessments occurred before and 1, 3, and 6 months into ETI therapy. Outcomes included change in percent predicted FEV1 (ppFEV1), sweat chloride concentration, body mass index (BMI), and self-reported respiratory symptoms.
Measurements and Main Results: Average age was 25.1 years, and 44.1% entered the study using tezacaftor/ivacaftor or lumacaftor/ivacaftor, whereas 6.7% were using ivacaftor, consistent with F508del homozygosity and G551D allele, respectively. At 6 months into ETI therapy, ppFEV1 improved 9.76 percentage points (95% confidence interval [CI], 8.76 to 10.76) from baseline, cystic fibrosis questionnaire-revised respiratory domain score improved 20.4 points (95% CI, 18.3 to 22.5), and sweat chloride decreased -41.7 mmol/L (95% CI, -43.8 to -39.6). BMI also significantly increased. Changes were larger in those naive to modulators but substantial in all groups, including those treated with ivacaftor at baseline.
Conclusions: ETI by clinical prescription provided large improvements in lung function, respiratory symptoms, and BMI in a diverse population naive to modulator drug therapy, using existing two-drug combinations, or using ivacaftor alone. Each group also experienced significant reductions in sweat chloride concentration, which correlated with improved ppFEV1 in the overall study population. Clinical trial registered with www.clinicaltrials.gov (NCT NCT04038047).
Dr David P Nichols is at the Department of Pediatrics, and. Cystic Fibrosis Foundation Therapeutics Development Network Coordinating Center, Seattle Children’s Research Institute, Seattle, Washington.
Megan E Gabel, Hongyue Wang, Daniel Gelfond, Christine Roach, Steven M Rowe, John P Clancy, Scott D Sagel, Drucy Borowitz, PROSPECT GIFT Sub-study Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network.Changes in Glucose Breath Test in Cystic Fibrosis Patients Treated with one Month of Lumacaftor/ivacaftor. J Pediatr Gastroenterol Nutr 2022 Apr 20.doi: 10.1097/MPG.0000000000003459.Online ahead of print.[Pubmed]

Megan E Gabel
Background:Alteration of the airway microbiota is a hallmark of cystic fibrosis (CF) pulmonary disease. Dysfunction of cystic fibrosis transmembrane regulator (CFTR) in the intestine also promotes changes in local microbiota such as small intestinal bacterial overgrowth (SIBO), which is common in CF. We evaluated whether therapy with the CFTR modulator combination lumacaftor/ivacaftor (luma/iva) has a beneficial impact on SIBO as measured by breath testing (BT).
Methods:A multicenter longitudinal study of CFTR-dependent disease profiling (NCT02477319) included a prospective evaluation for SIBO by BT. Tidal breath samples were collected after fasting and 15, 30, 45, 60, 90 and 120 minutes after ingestion of glucose, before and one month after subjects initiated luma + iva.
Results:Forty-two subjects enrolled in the sub-study (mean age = 23.3 years; 51% female; 9.5% Latinx); 38 completed a hydrogen BT at both time points, of which 73.7% had a positive BT prior to luma/iva (baseline) and 65.8% had a positive test after luma/iva (p = 0.44); shifts from negative to positive were also seen. Use of azithromycin (63.1%) and inhaled antibiotics (60.5%) were not associated with positive BT. Acid-blocking medications were taken by 73% of those with a negative BT at baseline and by 35% with a positive baseline BT (p = 0.04).
Conclusion:We found a high rate of positive hydrogen breath tests in individuals with CF, confirming that SIBO is common. One month of luma/iva did not significantly change the proportion of those with positive breath hydrogen measurements.
Megan E Gabelis a pediatric gastroenterologist in the Department of Pediatrics, University of Rochester School of Medicine, Rochester NY.
Rebecca Keyte, Sophia Kauser, Michail Mantzios, Helen Egan. The psychological implications and health risks of cystic fibrosis pre- and post- CFTR modulator therapy. Chronic Illn 2022 May 3;17423953221099042.doi: 10.1177/17423953221099042.Online ahead of print.[Pubmed]

Rebecca Keyte
Objectives: Cystic Fibrosis (CF) care is entering a period of personalised medicine with the emergence of CF transmembrane conductance regulator (CFTR) modulator therapies. Anecdotally individuals are reporting life-changing effects of modulator therapies, proposing an important area of study.
Methods: Twenty adult participants (males: 8, age range: 22-51 years, average FEV1: 53.45%) were recruited via social media to participate in a semi-structured interview; 17 participants were currently taking Elexacaftor/Tezacaftor/Ivacaftor (Kaftrio).
Results: An appreciation of a “normal life” post-modulator therapy is paramount, with improvements in symptoms and quality-of-life bringing a more urgent imperative for the provision of effective support to encourage positive health and lifestyle choices.
Discussion: In this new era of CF care, there remains many challenges present for the CF community, with participants suggesting that proactive psychological support is required along with proactive awareness regarding health risk behaviours for the current and future CF generations.
Rebecca Keyte is in the Department of Psychology, School of Social Sciences, 34651 Birmingham City University, Birmingham, UK.
Insa Korten, Elisabeth Kieninger, Linn Krueger, Marina Bullo, Christa E Flück, Philipp Latzin, Carmen Casaulta, Claudia Boettcher Short-Term Effects of Elexacaftor/Tezacaftor/Ivacaftor Combination on Glucose Tolerance in Young People With Cystic Fibrosis-An Observational Pilot Study. Front Pediatr 2022 Apr 21;10:852551.doi: 10.3389/fped.2022.852551.eCollection 2022.[Pubmed]

Insa Korten
The effect of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) on glucose tolerance and/or cystic-fibrosis-related diabetes (CFRD) is not well understood. We performed an observational study on the short-term effects of ELX/TEZ/IVA on glucose tolerance.
Methods:Sixteen adolescents with CF performed oral glucose tolerance tests (OGTT) before and 4-6 weeks after initiating ELX/TEZ/IVA therapy. A continuous glucose monitoring (CGM) system was used 3 days before until 7 days after starting ELX/TEZ/IVA treatment.
Results:OGTT categories improved after initiating ELX/TEZ/IVA therapy (p = 0.02). Glucose levels of OGTT improved at 60, 90, and 120 min (p < 0.05), whereas fasting glucose and CGM measures did not change.
Conclusion:Shortly after initiating ELX/TEZ/IVA therapy, glucose tolerance measured by OGTT improved in people with CF. This pilot study indicates that ELX/TEZ/IVA treatment has beneficial effects on the endocrine pancreatic function and might prevent or at least postpone future CFRD.
Insa Korten is in the Division of Paediatric Respiratory Medicine and Allergology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Karen S Raraigh, Michelle H Lewis, Joseph M Collaco, Mary Corey, Christopher M Penland, Anne L Stephenson, Johanna M Rommens, Carlo Castellani, Garry R Cutting. Caution advised in the use of CFTR modulator treatment for individuals harboring specific CFTR variants. J Cyst Fibros 2022 May 5;S1569-1993(22)00108-4.doi: 10.1016/j.jcf.2022.04.019.Online ahead of print [Pubmed]

Karen S Raraigh
In December 2020, the U.S. Food and Drug Administration (FDA) expanded the list of CFTR variants approved for treatment with CFTR modulators drugs from 39 to 183. Clinicians should be aware that individuals harboring certain variants approved for treatment may not respond to or benefit from this therapy. After review, the expert panel leading the CFTR2 project identified four categories of variants that may not result in a clinical response to modulator treatment: 15 variants assigned as non CF-causing; 45 variants of unknown significance; six variants known or suspected to cause mis-splicing as their primary defect rather than an amino acid substitution; and eight variants known to occur together in cis with another deleterious variant not expected to lead to CFTR protein (nonsense or frameshift). The potential risks and benefits of CFTR modulator therapy should be considered carefully for individuals harboring these variants.
Karen S Raraigh is a genetic counsellor at the McKusick-Nathans Department of Genetic Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States
Maya Desai, Chris Hine, Joanna L Whitehouse. Keith Brownlee, Susan C Charman, Prasad Nagakumar. Who are the 10%? – Non eligibility of cystic fibrosis (CF) patients for highly effective modulator therapies. Respir Med 2022 Aug;199:106878.doi: 10.1016/j.rmed.2022.106878. Epub 2022 May 16. [Pubmed]

Maya Desai
Background:The availability of mutation-specific cystic fibrosis modulator therapies has the potential to improve the lives of children and adults with cystic fibrosis. The frequency of mutations causing defects in the cystic fibrosis transmembrane conductance regulator (CFTR) function varies between sub-groups in multi-ethnic populations. The profile of patients eligible for CFTR modulator ivacaftor/tezacaftor/elexacaftor (Kaftrio™) therapy based on ethnicity has not been reported in the United Kingdom CF population.
Methods:We conducted a descriptive cross-sectional analysis of patients in the UK CF Registry who had annual review data submissions in 2019. Data analysed included demographic characteristics, spirometry, chronic Pseudomonas status, nutrition, and CF related diabetes status. The genotype data was stratified by whether there was at least one copy of F508del or no copy of F508del as current eligibility for ivacaftor/tezacaftor/elexacaftor, or projected future eligibility, is defined as having at least one copy of F508del mutation.
Results:Data from 9887 patients were reviewed, 46.7% female, mean age 22.5 years. 8.6% (n = 852) patients had no copy of F508del making them ineligible for ivacaftor/tezacaftor/elexacaftor. Overall, 93.4% of patients were of white ethnicity, with a similar proportion of those with at least one F508del being white (95.6%). This was reduced to 70.0% of those with no F508del. The proportion of people of Asian ethnicity was much higher in the no F508del group (19.2% vs 1.2%). Compared with one F508del patients, the no F508del group were older (25.2 years vs 22.2 years, p < 0.001), had higher prevalence of pancreatic sufficiency (39.0% vs 14.9% p < 0.001), lower prevalence of chronic Pseudomonas infection (21.1% vs. 26.6%, p < 0.001), and higher best FEV1from the previous year (proportion with greater than 70% FEV1 predicted, 66.1% vs 63.0%, p = 0.005).
Conclusion:Patients from black, Asian and minority ethnic backgrounds are significantly less likely to be eligible for ivacaftor/tezacaftor/elexacaftor based on the current prescribing policy in the UK. At present this is the most highly effective CF modulator therapy available to treat people with CF. The CF community should urgently address the unmet need for effective targeted therapies for patients without F508del.
Maya Desai is Respiratory Clinical Lead in the Department of Cystic Fibrosis and Respiratory Medicine, Birmingham Women’s and Children’s Hospital, Steelhouse Lane, Birmingham, UK.
Michael D Kim, Charles D Bengtson, Makoto Yoshida, Asef J Niloy, John S Dennis, Nathalie Baumlin, Matthias Salathe. Losartan ameliorates TGF-β1-induced CFTR dysfunction and improves correction by cystic fibrosis modulator therapies. J Clin Invest 2022 Jun 1;132(11):e155241. doi: 10.1172/JCI155241 Free PMC article [Pubmed]

Matthias Salthe
Highly effective modulator therapies dramatically improve the prognosis for those with cystic fibrosis (CF). The triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI) benefits many, but not all, of those with the most common F508del mutation in the CF transmembrane conductance regulator (CFTR).
Here, we showed that poor sweat chloride concentration responses and lung function improvements upon initiation of ETI were associated with elevated levels of active TGF-β1 in the upper airway. Furthermore, TGF-β1 impaired the function of ETI-corrected F508del-CFTR, thereby increasing airway surface liquid (ASL) absorption rates and inducing mucus hyperconcentration in primary CF bronchial epithelial cells in vitro. TGF-β1 not only decreased CFTR mRNA, but was also associated with increases in the mRNA expression of TNFA and COX2 and TNF-α protein. Losartan improved TGF-β1-mediated inhibition of ETI-corrected F508del-CFTR function and reduced TNFA and COX2 mRNA and TNF-α protein expression. This likely occurred by improving correction of mutant CFTR rather than increasing its mRNA (without an effect on potentiation), thereby reversing the negative effects of TGF-β1 and improving ASL hydration in the CF airway epithelium in vitro. Importantly, these effects were independent of type 1 angiotensin II receptor inhibition.
Corresponding author Matthias Salathe, pulmonologist in the Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
Francesca Saluzzo, Luca Riberi, Barbara Messore, Nicola Ivan Loré, Irene Esposito, Elisabetta Bignamini, Virginia De Rose. CFTR Modulator Therapies: Potential Impact on Airway Infections in Cystic Fibrosis. Cells 2022 Apr 6;11(7):1243.doi: 10.3390/cells11071243 Free PMC article [Pubmed]

Francesca Saluzzo
Cystic Fibrosis (CF) is an autosomal recessive disease caused by mutations in the gene encoding for the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) protein, expressed on the apical surface of epithelial cells. CFTR absence/dysfunction results in ion imbalance and airway surface dehydration that severely compromise the CF airway microenvironment, increasing infection susceptibility. Recently, novel therapies aimed at correcting the basic CFTR defect have become available, leading to substantial clinical improvement of CF patients. The restoration or increase of CFTR function affects the airway microenvironment, improving local defence mechanisms. CFTR modulator drugs might therefore affect the development of chronic airway infections and/or improve the status of existing infections in CF. Thus far, however, the full extent of these effects of CFTR-modulators, especially in the long-term remains still unknown.
This review aims to provide an overview of current evidence on the potential impact of CFTR modulators on airway infections in CF. Their role in affecting CF microbiology, the susceptibility to infections as well as the potential efficacy of their use in preventing/decreasing the development of chronic lung infections and the recurrent acute exacerbations in CF will be critically analysed.
Francesca Saluzzo is in the Emerging Bacterial Pathogens Unit, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, 20132 Milan, Italy.
Emily E Ricotta, D Rebecca Prevots, Kenneth N Olivier. CFTR modulator use and risk of nontuberculous mycobacteria positivity in cystic fibrosis, 2011-2018. ERJ Open Res 2022 Apr 11;8(2):00724-2021.doi: 10.1183/23120541.00724-2021.eCollection 2022 Apr. Free PMC article [Pubmed]

Emily Ricotta
Background: People with cystic fibrosis are at increased risk of pulmonary nontuberculous mycobacteria (NTM) disease. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators are associated with reduced lung infection with pathogens like Pseudomonas aeruginosa and Staphylococcus aureus. This association has not been studied with NTM.
Methods:Using encounter-level data from the US Cystic Fibrosis Foundation Patient Registry from 2011 to 2018, we identified individuals aged >12 years with one or more NTM-negative sputum culture and information on receipt of ivacaftor therapy. We used a Cox proportional hazards model to assess the relationship between CFTR modulator usage (any and monotherapy versus combination therapy) and NTM sputum culture positivity, controlling for sex, least severe class of CFTR mutation, receipt of chronic macrolides, age, body mass index and percentage predicted forced expiratory volume.
Results:Out of 25 987 unique individuals, 17 403 individuals met inclusion criteria. During follow-up, 42% of individuals received CFTR modulator therapy, and 23% had incident NTM. The median (interquartile range) time to event was 6.1 (4.0-7.3) years for those ever receiving CFTR modulators compared to 4.0 (1.6-6.5) years in those never receiving CFTR modulators. CFTR modulator use was associated with a significantly reduced hazard of NTM culture positivity (hazard ratio (HR) 0.88, 95% CI 0.79-0.97); there was no significant difference in the hazard between those receiving ivacaftor monotherapy versus combination therapy (combination HR 1.01, 95% CI 0.79-1.23).
Conclusions:CFTR modulator therapy is associated with a decreased risk of NTM positivity in individuals with cystic fibrosis.
Dr Emily E Ricotta is an epidemiologist in the Dept of Epidemiology and Population Studies Unit, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Bethesda, MD, USA.
Sylvia Szentpetery, Kimberly Foil, Sara Hendrix, Sue Gray, Christina Mingora, Barbara Head, Donna Johnson, Patrick A Flume. A case report of CFTR modulator administration via carrier mother to treat meconium ileus in a F508del homozygous fetus. J Cyst Fibros 2022 Apr 11;S1569-1993(22)00095-9.doi: 10.1016/j.jcf.2022.04.005.Online ahead of print.[Pubmed]

Sylvia Szentpetery
We report elexacaftor-tezacaftor-ivacaftor (ETI) treatment of a F508del carrier who was pregnant with a F508del homozygous fetus. At 23-weeks gestation meconium ileus (MI) was evident on ultrasound including dilated, hyperechoic bowel, which persisted on subsequent imaging. Through shared decision-making, the mother began ETI at 32 weeks with intent to treat fetal MI. The ultrasound findings persisted at treatment day 13, but bowel dilation had resolved by imaging on treatment day 27. A female infant was delivered vaginally at 36 weeks with no complications. The mother continued ETI while breastfeeding. Stool elastase at age 2 weeks was 240 mcg/g. Sweat chloride measurement was 64 and 62 mEq/L. Maternal and infant liver function testing have been normal. Maternal ETI treatment likely led to resolution of the MI and there is evidence supporting continued infant benefit through breastmilk. Logistical and ethical considerations regarding treatment of a carrier mother for infant benefit are discussed.
Dr Sylvia Szentpetery is a pediatric pulmonologist at the Medical University of South Carolina, Charleston, SC 29424, USA. Electronic address: szentpet@musc.edu.
Caitlyn Harvey, Sinead Weldon, Stuart Elborn, Damian G Downey, Clifford Taggart. The Effect of CFTR Modulators on Airway Infection in Cystic Fibrosis. Int J Mol Sci 2022 Mar 23;23(7):3513.doi: 10.3390/ijms23073513. [Pubmed]

The advent of Cystic fibrosis transmembrane receptor (CFTR) modulators in 2012 was a critical event in the history of cystic fibrosis (CF) treatment. Unlike traditional therapies that target downstream effects of CFTR dysfunction, CFTR modulators aim to correct the underlying defect at the protein level. These genotype-specific therapies are now available for an increasing number of CF patients, transforming the way we view the condition from a life-limiting disease to one that can be effectively managed.
Several studies have demonstrated the vast improvement CFTR modulators have on normalization of sweat chloride, CFTR function, clinical endpoints, and frequency of pulmonary exacerbation. However, their impact on other aspects of the disease, such as pathogenic burden and airway infection, remain under explored. Frequent airway infections as a result of increased susceptibility and impaired innate immune response are a serious problem within CF, often leading to accelerated decline in lung function and disease progression.
Current evidence suggests that CFTR modulators are unable to eradicate pathogenic organisms in those with already established lung disease. However, this may not be the case for those with relatively low levels of disease progression and conserved microbial diversity, such as young patients. Furthermore, it remains unknown whether the restorative effects exerted by CFTR modulators extend to immune cells, such as phagocytes, which have the potential to modulate the response of people with CF (pwCF) to infection. Throughout this review, we look at the potential impact of CFTR modulators on airway infection in CF and their ability to shape impaired pulmonary defences to pathogens.
Dr Caitlyn Harvey is in the Airway Innate Immunity Research (AiiR) Group, Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK.
Lena Wucherpfennig, Simon M F Triphan, Sabine Wege, Hans-Ulrich Kauczor, Claus P Heussel, Niclas Schmitt, Felix Wuennemann, Victoria L Mayer, Olaf Sommerburg, Marcus A Mall, Monika Eichinger, Mark O WielpützMagnetic resonance imaging detects improvements of pulmonary and paranasal sinus abnormalities in response to elexacaftor/tezacaftor/ivacaftor therapy in adults with cystic fibrosis. J Cyst Fibros 2022 Apr 7;S1569-1993(22)00088-1.: 10.1016/j.jcf.2022.03.011.Online ahead of print.[Pubmed]

Lena Wucherpfennig
Background: Therapy with Elexacaftor/Tezacaftor/Ivacaftor (ETI) was recently approved for adult cystic fibrosis (CF) patients with at least one F508del mutation. However, its effects on structural and functional lung abnormalities and chronic rhinosinusitis have not been studied by imaging.
Methods: 19 adults with CF (mean age 31±9y, range 19-55y) underwent standardized chest magnetic resonance imaging (MRI), and nine also same-session sinonasal MRI, before (MRI1) and after (MRI2) at least one month (mean duration 5 ± 3mon) on ETI. 24 control CF patients (30±7y, range 20-44y) without ETI underwent longitudinal chest MRI, and eleven also sinonasal MRI, twice (mean interval 40±15mon). MRI was assessed using the validated chest MRI score and chronic rhinosinusitis (CRS)-MRI score. Forced expiratory volume in 1 s percent predicted (FEV1%) was measured in all patients.
Results: In controls, the chest MRI global score and CRS-MRI sum score were stable from MRI1 to MRI2. In patients under ETI, the chest MRI global score improved (-11.4 ± 4.6, P<0.001), mainly due to reduction of bronchiectasis/wall thickening and mucus plugging subscores (-3.3 ± 2.2 and -5.2 ± 1.5, P<0.001, respectively). The improvement in chest MRI score correlated well with improved FEV1% (r=-0.703, P<0.001). The CRS-MRI sum score also improved in patients under ETI (-6.9 ± 3.0, P<0.001), mainly due to a reduction of mucopyoceles in the maxillary and ethmoid sinus (-50% and -39%, P<0.05, respectively).
Conclusions: MRI detects improvements of chest MRI and CRS-MRI scores in adult CF patients who first received ETI, demonstrating reversibility of structural lung and paranasal sinus abnormalities in patients with established disease.
Dr Lena Wucherpfennig is in the Department of Diagnostic and Interventional Radiology, Subdivision of Pulmonary Imaging, University Hospital Heidelberg, Im Neuenheimer Feld 420, 69120 Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC), German Center for Lung Research (DZL), Im Neuenheimer Feld 156, 69120 Heidelberg, Germany; Department of Diagnostic and Interventional Radiology with Nuclear Medicine, Thoraxklinik at University Hospital Heidelberg, Röntgenstr. 1, 69126 Heidelberg, Germany.
Clémence Martin, Martine Reynaud-Gaubert, Rebecca Hamidfar, Isabelle Durieu, Marlène Murris-Espin, Isabelle Danner-Boucher, Raphaël Chiron, Sylvie Leroy, Benoit Douvry, Dominique Grenet. Laurent Mely, Sophie Ramel, Sylvie Montcouquiol, LydieLemonnier, Espérie Burnet, Jean-Louis Paillasseur, Jennifer Da Silva, Pierre-Régis Burgel. Sustained effectiveness of elexacaftor-tezacaftor-ivacaftor in lung transplant candidates with cystic fibrosis. J Cyst Fibros. 2022 Feb 2;S1569-1993(22)00032-7.doi: 10.1016/j.jcf.2022.01.012. Online ahead of print [Pubmed]

Clemence Martin
Elexacaftor-tezacaftor-ivacaftor induces rapid clinical improvement in patients with cystic fibrosis (CF) and advanced pulmonary disease, often leading to suspend the indication for lung transplantation. Yet no long-term data is available in lung transplant candidates.
Methods: Lung transplant candidates (defined as being waitlisted for lung transplantation or considered for listing within 3 months) who have initiated elexacaftor-tezacaftor-ivacaftor were identified in the French cohort of patients with CF and advanced pulmonary disease. Patients were prospectively followed to evaluate treatment safety and effectiveness from initiation to July 20th, 2021.
Results: Among the 331 patients with advanced CF pulmonary disease who initiated elexacaftor-tezacaftor-ivacaftor, 65 were lung transplant candidates (17 listed for transplantation, 48 considered for listing within 3 months). Median [IQR] follow-up time was 363 [329; 377] days. At the end of the follow-up period, two patients were transplanted five and 11 days following treatment initiation, two were listed for transplantation, and 61 no longer met transplantation criteria. Improvement in percent predicted forced expiratory volume in 1 s (ppFEV1) at one month was +13.4% (95% confidence interval, 10.3%-16.5%; P < 0.0001) and remained stable thereafter. Treatment burden decreased substantially, with an 86% decrease in the need for intravenous antibiotics, 59% for oxygen therapy and 62% for non-invasive ventilation.
Conclusion: In lung transplant candidates eligible for elexacaftor-tezacaftor-ivacaftor, the rapid improvement following initiation of treatment persisted over one year with a reduction in treatment burden and lung transplantation could be safely deferred in most patients.
Clémence Martin is at the Université de Paris, Institut Cochin, InsermU1016, Paris, France; Respiratory Medicine and Cystic Fibrosis National Reference Center, Cochin Hospital, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France; ERN-Lung CF network, Frankfurt, Germany.
Gregory S Sawicki, Mark Chilvers, John McNamara, Lutz Naehrlich, Clare Saunders, Isabelle Sermet-Gaudelus, Claire E Wainwright, Neil Ahluwalia, Daniel Campbell, R Scott Harris, Hildegarde Paz-Diaz, Judy L Shih, Jane C Davies. A Phase 3, open-label, 96-week trial to study the safety, tolerability, and efficacy of tezacaftor/ivacaftor in children ≥ 6 years of age homozygous for F508del or heterozygous for F508del and a residual function CFTR variant. J Cyst Fibros 2022 Feb 18;S1569-1993(22)00033-9.doi: 10.1016/j.jcf.2022.02.003.Online ahead of print.[Pubmed]

Gregory Sawick
Background: Two previous Phase 3 studies (“parent studies”) showed that tezacaftor/ivacaftor was generally safe and efficacious for up to 24 weeks in children 6 through 11 years of age with cystic fibrosis (CF) and F508del/F508del (F/F) or F508del/residual function (F/RF) genotypes. We assessed the safety and efficacy of tezacaftor/ivacaftor in an open-label, 96-week extension study.
Methods: This was a Phase 3, 2-part, multicenter, open-label, extension study in children 6 through 11 years of age at treatment initiation (Study VX17-661-116; NCT03537651). The primary endpoint was safety and tolerability. Secondary endpoints were absolute change from baseline in lung clearance index2.5 (LCI2.5), sweat chloride (SwCl) concentration, Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score, and body mass index (BMI).
Results: One-hundred thirty children enrolled and received ≥ 1 dose of tezacaftor/ivacaftor; 109 completed treatment. Most (n = 129) had ≥ 1 treatment-emergent adverse event (TEAE), the majority of which were mild or moderate in severity and generally consistent with common manifestations of CF. Exposure-adjusted TEAE rates were similar to or lower than those in the parent studies. Five (3.8%) had TEAEs leading to treatment discontinuation. Efficacy results from the parent studies were maintained, with improvements in lung function, SwCl concentration, CFQ‑R respiratory domain score, and BMI observed from parent study baseline to Week 96.
Conclusions: Tezacaftor/ivacaftor is generally safe and well tolerated, and treatment effects are maintained for up to 120 weeks. These results support long-term use of tezacaftor/ivacaftor in children ≥ 6 years of age with CF and F/F or F/RF genotypes.
Dr Gregory Sawick is ta the Boston Children’s Hospital, Harvard Medical School, 300 Longwood Avenue, Boston, MA, USA.
Teresa Fuchs, Dorothea Appelt, Katharina Niedermayr, Helmut Ellemunter. REAL-world clinical effectiveness of ivacaftor therapy in the first 24 months in two infants with cystic fibrosis and different gating mutations-A case report. Clin Case Rep 2022 Feb 10;10(2):e05364.doi: 10.1002/ccr3.5364.eCollection 2022 Feb Free PMC article [Pubmed]
This study summarizes efficacy of ivacaftor treatment in 2 infants in a real-world setting. Two infants aged 2 and 11 months were started on ivacaftor off label with parental consent. A distinct decline of sweat chloride and lung clearance index plus increase in fecal elastase was seen. No side effects occurred. The results underline the early and sustainable effect in patients started aged less than 12 months and give cause for discussing whether a reduction in standard cystic fibrosis therapy is possible. Further details in the Free PMC article where other studies on young infants are discussed.
Teresa Fuchs is at the Medical University of Innsbruck Cystic Fibrosis Centre Innsbruck Innsbruck Austria
Marcel J C Bijvelds, Floris J M RoosKelly F Meijsen, Henk P Roest, Monique M A Verstegen, Hettie M Janssens, Luc J W van der LaanHugo R de Jonge. Rescue of chloride and bicarbonate transport by elexacaftor-ivacaftor-tezacaftor in organoid-derived CF intestinal and cholangiocyte monolayers. J Cyst Fibros 2022 May;21(3):537-543.doi: 10.1016/j.jcf.2021.12.006. Epub 2021 Dec 23. Free articl [Pubmed]

Marcel J C Bijuvelds
Background: In cystic fibrosis (CF), loss of CF transmembrane conductance regulator (CFTR)-dependent bicarbonate secretion precipitates the accumulation of viscous mucus in the lumen of respiratory and gastrointestinal epithelial tissues. We investigated whether the combination of elexacaftor (ELX), ivacaftor (IVA) and tezacaftor (TEZ), apart from its well-documented effect on chloride transport, also restores Phe508del-CFTR-mediated bicarbonate transport.
Methods: Epithelial monolayers were cultured from intestinal and biliary (cholangiocyte) organoids of homozygous Phe508del-CFTR patients and controls. Transcriptome sequencing was performed, and bicarbonate and chloride transport were assessed in the presence or absence of ELX/IVA/TEZ, using the intestinal current measurement technique.
Results: ELX/IVA/TEZ markedly enhanced bicarbonate and chloride transport across intestinal epithelium. In biliary epithelium, it failed to enhance CFTR-mediated bicarbonate transport but effectively rescued CFTR-mediated chloride transport, known to be requisite for bicarbonate secretion through the chloride-bicarbonate exchanger AE2 (SLC4A2), which was highly expressed by cholangiocytes. Biliary but not intestinal epithelial cells expressed an alternative anion channel, anoctamin-1/TMEM16A (ANO1), and secreted bicarbonate and chloride upon purinergic receptor stimulation.
Conclusions: ELX/IVA/TEZ has the potential to restore both chloride and bicarbonate secretion across CF intestinal and biliary epithelia and may counter luminal hyper-acidification in these tissues.
Marcel J C Bijvelds is a Principal Investigator in the Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, PO Box 2040, 3000CA Rotterdam, the Netherlands.
yment, Fadi Asfour, Margaret Rosenfeld, Mark Higgins, Lingyun Liu, Molly Mascia, Hildegarde Paz-Diaz, Simon Tian, Rachel Zahigian, Susanna A McColley, VX16-809-122 Study Group. A Phase 3, Open-Label Study of Lumacaftor/Ivacaftor in Children 1 to Less Than 2 Years of Age With Cystic Fibrosis Homozygous for F508del-CFTR. Am J Respir Crit Care Med 2022 Jun 30. doi: 10.1164/rccm.202204-0734OC.Online ahead of print.[Pubmed]

Jonathan Rayment
Rationale: Previous phase 3 trials showed treatment with lumacaftor/ivacaftor was safe and efficacious in people aged ≥2 years with cystic fibrosis homozygous for F508del-CFTR (F/F genotype).
Objectives: To assess the safety, pharmacokinetics, and pharmacodynamics of lumacaftor/ivacaftor in children aged 1 to <2 years with the F/F genotype.
Methods: This open-label, phase 3 study consisted of two parts (Part A [N = 14] and Part B [N = 46]) that enrolled two cohorts based on age (Cohort 1: 18 to <24 months and Cohort 2: 12 to <18 months). For the 15-day treatment period in Part A, lumacaftor/ivacaftor dose was based on weight at screening. Pharmacokinetic data from Part A were used to determine dose-based weight boundaries for Part B (24-week treatment period).
Measurements and main results: The primary endpoint of Part A was pharmacokinetics and the primary endpoint for Part B was safety and tolerability. Secondary endpoints for Part B were absolute change in sweat chloride concentration from baseline at Week 24 and pharmacokinetics. Analysis of pharmacokinetic data from Part A confirmed the appropriateness of Part B dosing. In Part B, 44 children (95.7%) had adverse events which for most were either mild (52.2% of children) or moderate (39.1% of children) in severity. The most common adverse events were cough, infective pulmonary exacerbation of cystic fibrosis, pyrexia, and vomiting. At Week 24, mean absolute change from baseline in sweat chloride concentration was ‒29.1 mmol/L (95% confidence interval, ‒34.8 to ‒23.4). Growth parameters (body mass index, weight, length, and associated z-scores) were normal at baseline and remained normal during the 24-week treatment period. Improving trends in some biomarkers of pancreatic function and intestinal inflammation such as fecal elastase-1, serum immunoreactive trypsinogen, and fecal calprotectin were observed.
Conclusions: Lumacaftor/ivacaftor was generally safe and well tolerated in children aged 1 to <2 years with the F/F genotype with a pharmacokinetic profile consistent with studies in older children. Efficacy results, including robust reductions in sweat chloride concentration, suggest the potential for CF disease modification with lumacaftor/ivacaftor treatment. These results support the use of lumacaftor/ivacaftor in this population
Dr Jonathan Rayment is paediatrician and researcher at the BC Children’s Hospital, 37210, Respiratory Medicine, Vancouver, British Columbia, Canada.
Claire E Wainwright A New Era for Cystic Fibrosis and CFTR Modulator Trials in Infants. Am J Respir Crit Care Med 2022 Jul 20.doi: 10.1164/rccm.202207-1356ED.Online ahead of print.[Pubmed]
(Summary as no abstract)

Claire Wainwright
As CFTR modulator therapies becomes established in young children a new approach to clinical care is likely to evolve as people with CF live longer lives ensuring healthy ageing will become a priority
In open label trials nearly 11% of children had transaminases elevation more than 3x upper limit of normal. Details of trails in young infants remain to be decided – open label trials appear to be extending to infants. There may be unrecognised effects on the young developing child and will open label trials be adequate to recognise these problems?
As CFTR modulation becomes established clinically the window for randomised placebo-controlled trials has almost closed. Consumers, clinicians and researchers need to rapidly determine how we move forward in developing new CFTR modulator therapy for infants and decide whether infants are indeed “little adults” and whether benefits can be effectively inferred or whether they deserve the same degree of evidence as older people expect?
Gimano D Amatngalim, Lisa W Rodenburg, Bente L Aalbers, Henriette Hm Raeven, Ellen M Aarts , Dounia Sarhane, Sacha Spelier, Juliet W Lefferts, Iris Al Silva, Wilco Nijenhuis, Sacha Vrendenbarg, Sabine Michel, Karin M de Winter-de Groot, Harry G Heijerman, Lukas C Kapitein, Magarida D Amaral, Cornelis K van der Ent, Jeffrey M Beekman. Measuring cystic fibrosis drug responses in organoids derived from 2D differentiated nasal epithelia. Life Sci Alliance 2022 Aug 3;5(12):e202101320.doi: 10.26508/lsa.202101320. Print 2022 [Pubmed
Cystic fibrosis is caused by genetic defects that impair the CFTR channel in airway epithelial cells. These defects may be overcome by specific CFTR modulating drugs, for which the efficacy can be predicted in a personalized manner using 3D nasal-brushing-derived airway organoids in a forskolin-induced swelling assay. Despite of this, previously described CFTR function assays in 3D airway organoids were not fully optimal, because of inefficient organoid differentiation and limited scalability. In this report, we therefore describe an alternative method of culturing nasal-brushing-derived airway organoids, which are created from an equally differentiated airway epithelial monolayer of a 2D air-liquid interface culture. In addition, we have defined organoid culture conditions, with the growth factor/cytokine combination neuregulin-1β and interleukin-1β, which enabled consistent detection of CFTR modulator responses in nasal-airway organoid cultures from subjects with cystic fibrosis.
Gimano D Amatngalim is in the Department of Pediatric Pulmonology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht University, Member of ERN-LUNG, Utrecht, The Netherlands and the University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands
Claire E Wainwright is Professor of Paediatrics and Child Health University of Queensland, Brisbane, Australia
Eva Choong,Alain Sauty, Angela Koutsokera, Sylvain Blanchon, Pascal André, Laurent Decosterd. Therapeutic Drug Monitoring of Ivacaftor, Lumacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis: Where Are We Now? Pharmaceutics 2022 Aug 11;14(8):1674.doi: 10.3390/pharmaceutics14081674Free PMC articl [Pubmed]

Eva Choong
Drugs modulating the cystic fibrosis transmembrane conductance regulator (CFTR) protein, namely ivacaftor, lumacaftor, tezacaftor, and elexacaftor, are currently revolutionizing the management of patients with cystic fibrosis (CF), particularly those with at least one F508delvariant (up to 85% of patients). These “caftor” drugs are mainly metabolized by cytochromes P450 3A, whose enzymatic activity is influenced by environmental factors, and are sensitive to inhibition and induction. Hence, CFTR modulators are characterized by an important interindividual pharmacokinetic variability and are also prone to drug-drug interactions. However, these CFTR modulators are given at standardized dosages, while they meet all criteria for a formal therapeutic drug monitoring (TDM) program that should be considered in cases of clinical toxicity, less-than-expected clinical response, drug or food interactions, distinct patient subgroups (i.e., pediatrics), and for monitoring short-term adherence.
While the information on CFTR drug exposure-clinical response relationships is still limited, we review the current evidence of the potential interest in the therapeutic drug monitoring caftor drugs in real-life settings.
Dr Eva Choong is Lab Deputy at Clinical Pharmacology, Department of Laboratory Medicine and Pathology, Lausanne University Hospital and University of Lausanne, 1011 Lausanne, Switzerland.
Lakshmi Viswanathan, Eric Bachman, Simon Tian, Neil Ahluwalia, Yaohua Zhang, Harold S Bernstein, Paul Panorchan. Phase 1 Study to Assess the Safety and Pharmacokinetics of Elexacaftor/Tezacaftor/Ivacaftor in Subjects Without Cystic Fibrosis With Moderate Hepatic Impairment. Eur J Drug Metab Pharmacokinet 2022 Aug 29.doi: 10.1007/s13318-022-00791-8.Online ahead of print[Pubmed]
Background and objective:Elexacaftor/tezacaftor/ivacaftor is highly effective in treating people with cystic fibrosis (pwCF) who have ≥ 1 responsive mutation. Liver disease occurs in approximately 10%-20% of pwCF. The objective of this study was to assess the safety and pharmacokinetics of elexacaftor/tezacaftor/ivacaftor in people with moderate hepatic impairment, which is necessary to inform on its use and guide dosing recommendations.
Methods:The safety and pharmacokinetics of elexacaftor/tezacaftor/ivacaftor were evaluated in subjects without CF with moderate hepatic impairment versus matched healthy controls. Twenty-two subjects (11 with moderate hepatic impairment and 11 healthy subjects) received half the standard adult daily dose of elexacaftor/tezacaftor/ivacaftor (elexacaftor 100 mg/tezacaftor 50 mg/ivacaftor 150 mg) orally for 10 days.
Results:Elexacaftor/tezacaftor/ivacaftor was safe and well tolerated in subjects with moderate hepatic impairment and healthy controls. On day 10, the mean values of the area under the curve during the dosing interval (AUCτ) for total (bound and unbound) elexacaftor and its major active metabolite M23-elexacaftor were increased 1.25-fold (95% CI 1.01, 1.54) and 1.73-fold (95% CI 1.27, 2.35), respectively, in subjects with moderate hepatic impairment compared with matched healthy subjects. The mean values of AUCτ for ivacaftor and tezacaftor were increased 1.50-fold (95% CI 1.09, 2.06) and 1.20-fold (95% CI 1.00, 1.43), respectively, while the mean value of AUCτfor the active metabolite M1-tezacaftor was 1.29-fold lower [ratio of moderate hepatic impairment to healthy subjects (95% CI): 0.778 (0.655, 0.924)] in subjects with moderate hepatic impairment.
Conclusions:A dose reduction of elexacaftor/tezacaftor/ivacaftor is warranted in people with moderate hepatic impairment.
Lakshmi Viswanathan is a clinical pharmacologist with Vertex Phamaceuticals in Boston USA
-I wonder if the non-CF patients were expected to improve. Were they informed it was not for their benefit? This paper is available in full on publisher’s website
Mitchell L Ramsey, Susan S Li, Luis F Lara, Yevgeniya Gokun, Venkata S Akshintala, Darwin L Conwell, John Heintz, Stephen E Kirkby, Karen S McCoy, Georgios I PapachristouAlpa Patel, Vikesh K Singh, Phil A Hart. Cystic fibrosis transmembrane conductance regulator modulators and the exocrine pancreas: A scoping review. J Cyst Fibros 2022 Aug 22;S1569-1993(22)00644-0.doi: 10.1016/j.jcf.2022.08.008.Online ahead of print.[Pubmed]
Background:Cystic fibrosis transmembrane conductance regulator (CFTR) modulators improve pulmonary outcomes in subjects with cystic fibrosis (CF); however, the effects on pancreatic manifestations are not well characterized. We hypothesized that CFTR modulators would improve measures of exocrine pancreatic function and outcomes.
Methods:We performed a systematic search to identify studies reporting measures of the exocrine pancreas in humans treated with CFTR modulators. Only studies reporting baseline and on-treatment assessments were included.
Results:Of 630 identified studies, 41 met inclusion criteria. CFTR modulators reduced acute pancreatitis events by 85% overall (rate ratio 0.15, 95% confidence interval (CI) 0.04, 0.52), with a greater effect seen in the subgroup with pancreas sufficient CF (PS-CF) (rate ratio 0.13 (95% CI 0.03, 0.53). Among 293 subjects with baseline and on-treatment evaluation of pancreas sufficiency, 253 were pancreas insufficient at baseline and 54 (21.3%) converted to pancreas sufficiency. Of 32 subjects with baseline FE-1 values <200 mcg/g, 16 (50%) increased to ≥200 mcg/g. Serum trypsin decreased by a mean of 565.9 ng/mL (standard deviation (SD) 311.8), amylase decreased by 38.2 U/L (SD 57.6), and lipase decreased by 232.3 U/L (SD 247.7).
Conclusions:CFTR modulator use reduces acute pancreatitis frequency and improves indirect measures of exocrine pancreas function. Future interventional studies that evaluate the mechanism and impact of CFTR modulators on acute pancreatitis and pancreas sufficiency in patients with CFTR dysfunction are warranted.Dr Mitchell L Ramsey is in the Division of Gastroenterology, Hepatology, and Nutrition, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Benjamin C Lyman, Jessica Seay, Casey Contreary, Adrienne P Savant, Mary Lynn Dell, George C Hescock. lexacaftor-tezacaftor-ivacaftor overdose in an adolescent female with cystic fibrosis. Pediatr Pulmonol 2022 Aug 12.doi: 10.1002/ppul.26116.Online ahead of print.[Pubmed]

Benjamin C Lyman
Summary of the letter to the Editor A 15-year-old female with CF (621+1G>T and F508del), pancreatic insufficiency, CF-related diabetes requiring insulin, and recurrent major depressive and anxiety disor e ders was started on ETI approximately 10 months before an intentional ingestion of an estimated 42 pills of ETI (two 1-week blister packs) in a suicide attempt. Less than 1 h post-ingestion, she self-induced emesis, then presented to the emergency department (ED), where she also complained of increased sputum production consistent with a pulmonary exacerbation. Vital signs and physical exam were within normal limits for age. Complete blood count, comprehensive metabolic panel (CMP), coagulation markers, urine drug screen, salicylate, acetaminophen, ethanol, and electrocardiogram were within normal limits.
Maximum elevation in LFTs was seen at 12-day postingestion and noted to be 5.7 and 4.1 times the upper limit of normal for AST and ALT, respectively. Given the primary concern of liver damage with ETI toxicity, the patient’s ETI dose was held for 9 days. On day 10 postingestion, following LFT reduction to less than twice the upper limit of normal, the two pill, morning dose (ETI: 100-50-75 mg) was restarted. Her LFTs had an acute rise on day 12 postingestion; therefore, ETI was held for an additional day. On day 14 postingestion, the morning dose was again restarted. LFTs returned to normal range by day 19 postingestion. The nighttime dose (ivacaftor 150 mg) was restarted on day 28 postingestion, after sustained stabilization of LFTs..
In conclusion, as care for pwCF enters the modulator era, it is critical to partner with mental health providers, screen pwCF for mental health issues, and recognize the potential for worsening of mental health with modulators.
Katharina Niedermayr, Verena Gasser, Claudia Rueckes-Nilges, Dorothea Appelt, Johannes Eder, Teresa Fuchs, Lutz Naehrlich, Helmut Ellemunter. Personalized medicine with drugs targeting the underlying protein defect in cystic fibrosis: is monitoring of treatment response necessary. Ther Adv Chronic Dis 2022 Aug 5;13:20406223221108627.doi: 10.1177/20406223221108627.eCollection 2022.Free PMC article [Pubmed]
Cystic fibrosis (CF) is caused by two mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. In the last years, drugs targeting the underlying protein defect like lumacaftor/ivacaftor (LUM/IVA) or tezacaftor/ivacaftor (TEZ/IVA) and more recently elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) were admitted. Outcome parameters evaluating therapy response like forced expiratory pressure in 1 s (FEV1), body mass index (BMI) or the efficacy of CFTR function in sweat glands showed improvement in several cases. Other, CFTRbiomarkers were analysed rarely.
This prospective observational study was aimed at evaluating CFTR function in patients treated with different CFTR modulators together with common valid clinical outcome parameters at standardized appointments (day 0, week 2, 4, 16). We followed four patients with the same mutation (F508del-CFTR), sex, age and disease severity. Monitoring focused on lung function, gastrointestinal aspects and CFTR function of sweat glands, nasal and intestinal epithelium. Sweat tests were performed by pilocarpine iontophoresis. Nasal potential difference (NPD) measured transepithelial voltage in vivo and potential increased when CFTRfunction improved. Rectal biopsies were obtained for intestinal current measurements (ICM) ex vivo. Intestinal CFTR function was assessed by stimulating chloride secretion with different reagents.
Response to CFTR modulators regarding clinical outcome parameters was rather variable. A sweat chloride reduction of 35.3 mmol/L, nasal CFTR rescue of 4.4% and fivefold higher CFTR function in the intestine was seen at week 16 post-LUM/IVA. Due to our monitoring, we identified a non-responder to LUM/IVA and TEZ/IVA. In case of ELX/TEZ/IVA, clinical parameters and CFTR bioassays improved and were concordant. Although our cohort is small, results emphasize that non-responders exist and conclusions could not be drawn if patients were not monitored. Data on CFTR function can confirm or disprove ongoing CFTR dysfunction and might be helpful selectively. Non-responders need other alternative therapy options as demonstrated with ELX/TEZ/IVA.
Dr Katharina Niedermayr is in the Department for Child and Adolescent Health, University Clinic for Paediatrics III, Cystic Fibrosis Centre, Medical University of Innsbruck, Anichstrasse 35, 6020 Innsbruck, Tyrol, Austria.
Bethany Collins, Christopher Fortner, Amanda Cotey, Charles R Jr Esther, Aaron Trimble. Drug exposure to infants born to mothers taking Elexacaftor, Tezacaftor, and Ivacaftor. J Cyst Fibros 2022 Jul;21(4):725-727.doi: 10.1016/j.jcf.2021.12.004. Epub 2021 Dec 22.[Pubmed]

Bethany Collins ousu.edu
Elexacaftor, tezacaftor, ivacaftor (ETI) have been associated with marked clinical improvements in adults with CF, which appears to be associated with increased fertility. However, maternal and fetal effects of therapy continued during pregnancy are not well understood. We collected maternal blood, infant blood, cord blood, and breast milk from 3 mother-infant pairs from women who elected to remain on ETI therapy while pregnant. Our results demonstrated relatively high levels of ETI in cord blood, suggesting placental transfer of these compounds, as well as low levels of ETI in breast milk and infant blood, suggesting further transfer of these compounds to breast-fed infants in the postnatal period. These data underscore the need for larger studies on the effects of modulator surrounding reproduction.
Bethany Collins is in the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Oregon Health & Science University.
Brionna N Hudson, Hollyann R Jacobs , Alexander Philbrick, Xiaolong A Zhou, Michelle M Simonsen, Julie A Safirstein, Shannon M Rotolo. Drug-induced acne with elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis. J Cyst Fibros 2022 Sep 7;S1569-1993(22)00657-9.doi: 10.1016/j.jcf.2022.09.002.Online ahead of print. [Pubmed]

Brianna S Hudson linkedin,com
Elexacaftor/tezacaftor/ivacaftor (ELX-TEZ-IVA) is a Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator shown to improve lung function and reduce sweat chloride in people with Cystic Fibrosis (CF). The only commonly reported dermatologic adverse effect with CFTR modulators including ELX-TEZ-IVA is rash. In this case series, we describe 19 patients who reported new onset or worsening of acne after initiation of this drug to their CF pharmacist or another member of their CF care team. The mechanism and frequency of this adverse effect is unknown.
Dr Brionna N Hudson is at the College of Pharmacy Chicago State University
Sylvia Szentpetery, Kimberly Foil, Sara Hendrix, Sue Gray, Christina Mingora, Barbara Head, Donna Johnson, Patrick A Flume. A case report of CFTR modulator administration via carrier mother to treat meconium ileus in a F508del homozygous fetus. J Cyst Fibros 2022 Apr 11;S1569-1993(22)00095-9.doi: 10.1016/j.jcf.2022.04.005.Online ahead of print.[Pubmed]

Sylvia Szentpetery
education.musc.edu
We report elexacaftor-tezacaftor-ivacaftor (ETI) treatment of a F508del carrier who was pregnant with a F508del homozygous fetus. At 23-weeks gestation meconium ileus (MI) was evident on ultrasound including dilated, hyperechoic bowel, which persisted on subsequent imaging. Through shared decision-making, the mother began ETI at 32 weeks with intent to treat fetal MI. The ultrasound findings persisted at treatment day 13, but bowel dilation had resolved by imaging on treatment day 27. A female infant was delivered vaginally at 36 weeks with no complications. The mother continued ETI while breastfeeding. Stool elastase at age 2 weeks was 240 mcg/g. Sweat chloride measurement was 64 and 62 mEq/L. Maternal and infant liver function testing have been normal. Maternal ETI treatment likely led to resolution of the MI and there is evidence supporting continued infant benefit through breastmilk. Logistical and ethical considerations regarding treatment of a carrier mother for infant benefit are discussed.
Dr Sylvia Szentpetery is a pediatric pulmonologist at the Medical University of South Carolina, Charleston, SC 29424, USA. Electronic address: szentpet@musc.edu.
Lauryn A Benninger, Cesar Trillo, Jorge Lascano. CFTR modulator use in post lung transplant recipients. J Heart Lung Transplant 2021 Dec;40(12):1498-1501.doi: 10.1016/j.healun.2021.08.009.Epub 2021 Aug 26. [Pubmed]

Lauryn Benninger researchgate.net
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator therapy has previously been contraindicated in solid organ transplant recipients. This was due to lack of data and concern for interactions with immunosuppressive drug regimens. However, in post-lung transplant recipients, CFTR modulators may improve extrapulmonary manifestations of cystic fibrosis without impacting graft function or immunosuppressive drug levels. Herein, we present our single center experience with the use of elexacaftor/tezacaftor/ivacaftor, Trikafta, in adult post-lung transplant recipients.
Dr Lauryn A Benninger is a pulmonologist in the Department of Internal Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of Florida College of Medicine, Gainesville, Florida
Ann Cheng, Olivia Baker, Uta Hill. Elexacaftor, tezacaftor and ivacaftor: a case of severe rash and approach to desensitisation. BMJ Case Rep 2022 Mar 2;15(3):e247042. doi: 10.1136/bcr-2021-247042. pubmed.ncbi.nlm.nih.gov/35236685

Ann Cheng uk.linkedin.com
We present a case of severe rash following induction of elexacaftor, tezacaftor and ivacaftor (ELX/TEZ/IVA) in a young adult male cystic fibrosis patient. While rash is a commonly reported side effect which resolves in 1-2 weeks with minimal intervention, our patient had presented with fever and widespread rash prompting medication cessation. After a washout period, reintroduction with 1/2 tablet of ELX/TEZ/IVA produced a similar systemic response within 24 hours. Repeat attempt, this time with 1/8 tablet and increasing in increments of an eighth daily, was successful and has allowed our patient to experience the transformative benefits of ELX/TEZ/IVA including improved pulmonary function and reduced episodes of infective exacerbation. This case illustrates one of the most common side effects of ELX/TEZ/IVA triple therapy, and our experience of desensitisation to ELX/TEZ/IVA in a challenging case of rash.
Dr Ann Cheng is NIHR Academic Clinical Fellow in the Department of Infection, Immunity and Cardiovascular Disease, The University of Sheffield, Sheffield, UK. Also Cambridge Centre for Lung Infection, Royal Papworth Hospital, Cambridge, UK.
Eva Choong, Alain Sauty , Angela Koutsokera , Sylvain Blanchon , Pascal André , Laurent Decosterd. Therapeutic Drug Monitoring of Ivacaftor, Lumacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis: Where Are We Now? Pharmaceutics 2022 Aug 11;14(8):1674.doi: 10.3390/pharmaceutics14081674 Free PMC article pubmed.ncbi.nlm.nih.gov/36015300/

Eva Choong ch.linkedin.com
Drugs modulating the cystic fibrosis transmembrane conductance regulator (CFTR) protein, namely ivacaftor, lumacaftor, tezacaftor, and elexacaftor, are currently revolutionizing the management of patients with cystic fibrosis (CF), particularly those with at least one F508delvariant (up to 85% of patients). These “caftor” drugs are mainly metabolized by cytochromes P450 3A, whose enzymatic activity is influenced by environmental factors, and are sensitive to inhibition and induction. Hence, CFTR modulators are characterized by an important interindividual pharmacokinetic variability and are also prone to drug-drug interactions. However, these CFTR modulators are given at standardized dosages, while they meet all criteria for a formal therapeutic drug monitoring (TDM) program that should be considered in cases of clinical toxicity, less-than-expected clinical response, drug or food interactions, distinct patient subgroups (i.e., pediatrics), and for monitoring short-term adherence.
While the information on CFTR drug exposure-clinical response relationships is still limited, we review the current evidence of the potential interest in the therapeutic drug monitoring caftor drugs in real-life settings.
Dr Eva Choong is Lab Deputy at Clinical Pharmacology, Department of Laboratory Medicine and Pathology, Lausanne University Hospital and University of Lausanne, 1011 Lausanne, Switzerland.
Hanna Crow , Charles Bengtson, Xiaosong Shi , Leland Graves 3rd , Abeer Anabtawi. CGM (continuous glucose monitoring)patterns in adults with cystic fibrosis-related diabetes before and after elexacaftor-tezacaftor-ivacaftor therapy. J Clin Transl Endocrinol 2022 Oct 1;30:100307. doi: 10.1016/j.jcte.2022.100307. eCollection 2022 Dec. pubmed.ncbi.nlm.nih.gov/36217440

Hanna Crow
health.usnews.com
Cystic fibrosis-related diabetes (CFRD) is a common complication of cystic fibrosis that is associated with worse outcomes and higher mortality rates. CF transmembrane conductance regulator gene (CFTR) modulators have shown favorable effects on lung function, pulmonary exacerbations, and nutrition status. However, data regarding effects of CFTR modulators on glycemic control among those with CFRD is lacking. In this retrospective study, CGM data was analyzed to determine effect of elexacaftortezacaftor- ivacaftor therapy (ETI), a CFTR modulator, on glucose control among patients with CFRD. No difference was seen in glucose patterns after 3- and 6- months of starting ETI.
Hanna Crow is an endocrinologist, diabetes, metabolism and internal medicine at the Department of Internal medicine, Kansas Medical Center.
Samantha L Durfey, Sudhakar Pipavath, Anna Li, Anh T Vo, Anina Ratjen, Suzanne Carter, Sarah J Morgan, Matthew C Radey, Brenda Grogan, Stephen J Salipante, Michael J Welsh, David A Stoltz, Christopher H Goss , Edward F McKone , Pradeep K Singh./ Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections. mBio 2021 Dec 21;12(6):e0314821. doi: 10.1128/mbio.03148-21.Epub 2021 Dec 14. Free PMC article [Pubmed]

Samantha Durfey depts.washington.edu
Drugs called CFTR modulators improve the physiologic defect underlying cystic fibrosis (CF) and alleviate many disease manifestations. However, studies to date indicate that chronic lung infections that are responsible for most disease-related mortality generally persist.
Here, we investigated whether combining the CFTR modulator ivacaftor with an intensive 3.5-month antibiotic course could clear chronic Pseudomonas aeruginosa or Staphylococcus aureus lung infections in subjects with R117H-CFTR, who are highly ivacaftor-responsive. Ivacaftor alone improved CFTR activity, and lung function and inflammation within 48 h, and reduced P. aeruginosa and S. aureus pathogen density by ∼10-fold within a week. Antibiotics produced an additional ∼10-fold reduction in pathogen density, but this reduction was transient in subjects who remained infected. Only 1/5 P. aeruginosa-infected and 1/7 S. aureus-infected subjects became persistently culture-negative after the combined treatment. Subjects appearing to clear infection did not have particularly favourable baseline lung function or inflammation, pathogen density or antibiotic susceptibility, or bronchiectasis scores on CT scans, but they did have remarkably low sweat chloride values before and after ivacaftor. All persistently P. aeruginosa-positive subjects remained infected by their pre-treatment strain, whereas subjects persistently S. aureus-positive frequently lost and gained strains. This work suggests chronic CF infections may resist eradication despite marked and rapid modulator-induced improvements in lung infection and inflammation parameters and aggressive antibiotic treatment.
IMPORTANCE Recent work shows that people with CF and chronic lung infections generally remain persistently infected after treatment with drugs that target the CF physiological defect (called CFTR modulators). However, changes produced by modulators could increase antibiotic efficacy. We tested the approach of combining modulators and intensive antibiotics in rapid succession and found that while few subjects cleared their infections, combined treatment appeared most effective in subjects with the highest CFTR activity. These findings highlight challenges that remain to improve the health of people with CF.
Dr Samantha L Durfey is in the Department of Microbiology, University of Washington School of Medicinegrid.471394.c, Seattle, Washington, USA.
Hannah Farley, Sarah Poole, Stephen Chapman, William Flight. Diagnosis of cystic fibrosis in adulthood and eligibility for novel CFTR modulator therapy. Postgrad Med J 2022 May;98(1159):341-345.doi: 10.1136/postgradmedj-2020 pubmed.ncbi.nlm.nih.gov/33452147/

Hannah Farley univ.ox.ac.uk
Background: Cystic fibrosis (CF) is an autosomal recessive condition that primarily manifests as a chronic respiratory disease. CF is usually diagnosed in early childhood or through newborn screening although in a small but important group, diagnosis is not made until adulthood. Highly effective cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies are now available for most genetic causes of CF highlighting the importance of identifying people with late presentations of CF.
Aim: We aimed to identify the clinical characteristics of people diagnosed with CF in adulthood and their resulting eligibility for novel CFTR modulator therapies.
Methods: Patients diagnosed with CF at age 18 years or older were identified from a patient database. Paper and electronic medical records were reviewed and clinical, microbiological and radiological data at diagnosis were recorded.
Results: Nineteen patients were identified. Median age at diagnosis was 38 years (range: 19-71) and 9 (47%) were female. All patients had a history of chronic respiratory symptoms and 18/19 (94%) had radiological evidence of bronchiectasis. All patients had two pathogenic CFTR mutations identified with 16/19 (84%) compound heterozygotes for the F508del mutation. The majority of patients had a CFTR genotype considered eligible for CFTR modulator therapy (84% and 89% according to European and US licences, respectively).
Conclusions: Adult patients with unexplained chronic bronchiectasis should be thoroughly investigated for CF. A low index of suspicion will help to identify adults with undiagnosed CF who are likely to benefit from CFTR modulator therapy.
Dr Hannah Farley is at the Medical School, Oxford University, Oxford, UK
Thomas S FitzMaurice, Caroline McCann, Dilip Nazareth, Matthew Shaw, Paul S McNamara, Martin J Walshaw. Measuring the effect of elexacaftor/tezacaftor/ivacaftor combination therapy on the respiratory pump in people with CF using dynamic chest radiography. J Cyst Fibros 2022 Jan 28;S1569-1993(22)00027-3. doi: 10.1016/j.jcf.2022.01.007.Online ahead of print. [Pubmed]

Thomas S FitzMaurice researchgate.net
Background: The CFTR modulator elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) leads to significant improvement in the symptoms and spirometry of people with cystic fibrosis (pwCF), but little evidence exists to understand its effect on respiratory pump function. Dynamic chest radiography (DCR) is a novel cineradiographic tool that identifies and tracks the chest wall and diaphragm throughout the breathing cycle, alongside fluoroscopic images of the chest of diagnostic quality.
Methods: In this observational work, we examined the spirometry and DCR of 24 pwCF before and after starting ELX/TEZ/IVA. DCR automatically tracked the hemidiaphragm midpoints and projected lung area (PLA) during tidal and deep breathing manoeuvres.
Results: ppFEV1 (61±18 to 73±22, P<0.001) and ppFVC (77±16 to 88±15, P<0.001) improved significantly. DCR demonstrated a significant increase in hemidiaphragm excursion on both the right (18±11 to 26±9 mm, P<0.001) and left (21±11 to 31±11 mm, P<0.001) sides, as well as maximum hemidiaphragm speed during inspiration (right 22±14 to 31±11 mm/s, P=0.03; left 28±11 to 37±16 mm/s, P=0.02). PLA at end-expiration was significantly reduced (334±71 to 290±72cm2, P<0.001), with a significant increase in ΔPLA (83±40 to 117±36cm2, P<0.001).
Conclusions: DCR demonstrated significant improvements in hemidiaphragm excursion and ΔPLA in pwCF started on ELX/TEZ/IVA. These changes likely reflect a reduction in air trapping and improved elastic recoil of the chest, and are consistent with improvements seen in spirometry. The changes seen with DCR are physiologically plausible and correlate well with spirometry. DCR warrants further investigation as a tool for assessing the impact of CFTR-modulating therapies.
Dr Thomas S FitzMaurice is a Fellow in the Adult CF Unit, Liverpool Heart and Chest Hospital, Thomas Drive, Liverpool L14 3PE, UK; Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK.
Thomas Simon FitzMaurice, Dilip Nazareth, Kapil Iyer, Martin Walshaw, Mohamed Al-Aloul. Elexacaftor/Tezacaftor/Ivacaftor as a Bridge to Lung Retransplant in a Recipient With Cystic Fibrosis. Exp Clin Transplant 2022 Mar 15.doi: 10.6002/ect.2021.0468.Online ahead of print. Free article [Pubmed]
The triple-combination cystic fibrosis transmembrane conductance regulator modulator elexacaftor/- tezacaftor/ivacaftor is known to improve lung function and have extrapulmonary benefits in people with cystic fibrosis. However, there is limited evidence for its use in patients with cystic fibrosis after lung transplant, where the donor lung expresses normal levels of the cystic fibrosis transmembrane conductance regulator. We describe the use of elexacaftor/tezacaftor/ivacaftor as a bridge to potential lung retransplant in a 37-year-old man with cystic fibrosis and chronic lung allograft dysfunction. Although forced expiratory volume in 1 second did not improve, the patient had decreased sputum volume, no pulmonary exacerbations of cystic fibrosis, and no longer required continuous antibiotic therapy. Pancreatic function, revised Cystic Fibrosis Questionnaire scores, sinus symptoms, weight, and corticosteroid dependence significantly improved. There were no reported side effects attributable to elexacaftor/tezacaftor/ivacaftor. However, the patient exhibited declined renal function, which had been initially attributed to lability in cyclosporin levels but which were corrected after lithotripsy for renal calculi.
Triple-combination modulators of the cystic fibrosis transmembrane conductance regulator may offer benefits to carefully selected individuals awaiting retransplant, balanced against the risk of worsened immunosuppressant level control.
Thomas Simon FitzMaurice is a Fellow in the Adult CF Unit, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom and the Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom.
Teresa Fuchs, Dorothea Appelt, Katharina Niedermayr, Helmut Ellemunter. REAL-world clinical effectiveness of ivacaftor therapy in the first 24 months in two infants with cystic fibrosis and different gating mutations-A case report. Clin Case Rep 2022 Feb 10;10(2):e05364.doi: 10.1002/ccr3.5364.eCollection 2022 Feb Free PMC article [Pubmed]
This study summarizes efficacy of ivacaftor treatment in 2 infants in a real-world setting. Two infants aged 2 and 11 months were started on ivacaftor off label with parental consent. A distinct decline of sweat chloride and lung clearance index plus increase in fecal elastase was seen. No side effects occurred. The results underline the early and sustainable effect in patients started aged less than 12 months and give cause for discussing whether a reduction in standard cystic fibrosis therapy is possible. Further details in the Free PMC article where other studies on young infants are discussed.
Teresa Fuchs is at the Medical University of Innsbruck Cystic Fibrosis Centre Innsbruck Innsbruck Austria
Michelle J Gould, Haley Smith, Jonathan H Rayment, Helen Machida, Tanja Gonska, Gary J Galante. CFTR modulators increase risk of acute pancreatitis in pancreatic insufficient patients with cystic fibrosis. J Cyst Fibros 2022 Jul;21(4):600-602.doi: 10.1016/j.jcf.2021.09.010. Epub 2021 Oct 31. [Pubmed]

Michelle Gould mobile.twitter.com
Patients with pancreatic insufficient cystic fibrosis rarely develop acute pancreatitis due to insufficient acinar reserve. We describe a series of five patients under the age of 18 (range 8-16 years) with pancreatic insufficient cystic fibrosis who developed a phenotype in keeping with acute pancreatitis following initiation of CFTR modulator therapy. This occurred at a median of 30 months following CFTR modulator initiation. 3/5 of these patients also developed pancreatic sufficiency or at least an intermediary pancreas status, indicated by fecal elastases above 100 μg/g.
This series highlights a mostly unrecognized potential side effect of this therapy as well as the potential of CFTR modulator therapies to improve exocrine pancreatic function, even in adolescent patients.
Michelle J Gould is in the Department of Pediatrics, University of Toronto, Toronto, Ontario, Canada.
Simon Y Graeber, Diane M Renz, Mirjam Stahl, Sophia T Pallenberg, Olaf Sommerburg , Lutz Naehrlich, and 16 others. Effects of Elexacaftor/Tezacaftor/Ivacaftor Therapy on Lung Clearance Index and Magnetic Resonance Imaging in Patients with Cystic Fibrosis and One or Two F508del Alleles. Am J Respir Crit Care Med 2022 May 10.doi: 10.1164/rccm.202201-0219OC. Online ahead of print. [Pubmed]

Simon Graeber researchgate.net
Rationale: We recently demonstrated that triple combination CFTR modulator therapy with elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) improves CFTR function in airway and intestinal epithelia to 40 to 50% of normal in patients with cystic fibrosis (CF) with one or two F508del alleles. In previous studies, this improvement of CFTR function was shown to improve clinical outcomes, however, effects on the lung clearance index (LCI) determined by multiple breath washout and abnormalities in lung morphology and perfusion detected by magnetic resonance imaging (MRI) have not been studied.
Objectives: To examine the effect of ELX/TEZ/IVA on LCI and lung MRI scores in patients with CF and one or two F508del alleles aged 12 years and older.
Methods: This prospective, observational, multicenter, post-approval study assessed LCI and lung MRI scores before and 8-16 weeks after initiation of ELX/TEZ/IVA.
Measurements and main results: A total of 91 patients with CF including 45 heterozygous for F508del and a minimal function mutation (MF) and 46 homozygous for F508del were enrolled in this study. Treatment with ELX/TEZ/IVA improved LCI in F508del/MF (-2.4;IQR, -3.7 – -1.1;P<0.001) and F508del homozygous (-1.4;IQR, -2.4 – -0.4;P<0.001) patients. Further, ELX/TEZ/IVA improved the MRI global score in F508del/MF (-6.0;IQR, -11.0 – -1.3;P<0.001) and F508del homozygous (-6.5;IQR, -11.0 – -1.3;P<0.001) patients.
Conclusions: Our data demonstrate that improvement of CFTR function by ELX/TEZ/IVA improves lung ventilation and abnormalities in lung morphology including airway mucus plugging and wall thickening in adolescent and adult patients with CF and one or two F508del alleles in a real-world, post-approval setting.
Simon Y Graeber is at the Charité Universitätsmedizin Berlin, 14903, Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine and Cystic Fibrosis Center, Berlin, Germany, the Berlin Institute of Health at Charité, 522475, Berlin, Germany and the German Center for Lung Research, 542891, associated partner site, Berlin, Germany
Barbara R Grubb, Alessandra Livraghi-Butrico. Animal models of cystic fibrosis in the era of highly effective modulator therapies. Curr Opin Pharmacol 2022 Jun;64:102235. doi: 10.1016/j.coph.2022.102235.Epub 2022 May 13. [Pubmed]

Alessandra Livraghi-Butrico med.unc.edu

Barbara Grubb med.unc.edu
Few human genetic diseases can rely on the availability of as many and as diverse animal models as cystic fibrosis (CF), a multiorgan syndrome caused by functional absence of cystic fibrosis transmembrane regulator (CFTR). The recent development of highly effective CFTR modulator drug therapies simultaneously highlighted the remarkable clinical improvement achievable with these treatments, the lack of therapeutic alternatives for non-responders, and the need to understand the kinetics of disease upon early life/chronic treatment. These advances have rekindled efforts to leverage animal models to address critical knowledge gaps in CF. This article provides a concise overview of the areas of interests for therapeutic intervention in the current CF landscape, focusing on the contributions of in vivo models to understand CF pathogenesis, identify therapeutic windows, and develop novel therapies for all CFTR mutations.
Both authors are at the Marsico Lung Institute, University of North Carolina School of Medicine, Chapel Hill, NC, 27599, USA.
Jonathan Guo, Anna Garratt, Andrew Hill. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros 2022 Feb 3;S1569-1993(22)00031-5.doi: 10.1016/j.jcf.2022.01.009.Online ahead of print. [Pubmed]
Jonathan Guo uk.linkedin.com
Background: Time has seen management for Cystic Fibrosis (CF) advance drastically, most recently in the development of the disease-modifying triple combination therapy ivacaftor/tezacaftor/elexacaftor. There is currently limited evidence regarding both the global epidemiology of CF and access to this transformative therapy – and therefore where needs are not being met. Therefore, this study aims to define gaps in access to CF treatment.
Methods: Patient data were extracted from established CF registries. Where these were not available, literature searches were conducted alongside an international survey of 51 CF experts to determine the diagnosed patient population. National CF prevalence estimates were combined with registry data on estimated population coverage, to extrapolate the total estimated number of undiagnosed patients. Estimates of ivacaftor/tezacaftor/elexacaftor treatment coverage were extracted from publicly available sales summaries and pricing data.
Results: 162,428 [144,606-186,620] people are estimated to be living with CF across 94 countries. Of these, an estimated 105,352 (65%) are diagnosed, with 19,516 (12%) receiving triple combination therapy. We estimated 57,076 patients with undiagnosed CF. Owing to a paucity of high-quality data, estimates of undiagnosed CF in low- and middle-income countries are highly uncertain. Patient registries were available in 45 countries, and used to identify 90% of the estimated diagnosed population.
Conclusions: A significant CF patient burden exists in countries where disease-modifying drugs are unavailable, and final figures are likely underestimates. This analysis shows the potential to improve rates of diagnosis and treatment for CF, so a higher percentage of patients receive the most effective triple combination treatment.
Dr Jonathan Guo is in the Faculty of Medicine, Imperial College London, United Kingdom.
Jonathan Guo , Junzheng Wang , Jingchun Zhang , Joseph Fortunak , Andrew Hill Current prices versus minimum costs of production for CFTR modulators. J Cyst Fibros. 2022 Sep;21(5):866-872. doi: 10.1016/j.jcf.2022.04.007. Epub 2022 Apr 16 pubmed.ncbi.nlm.nih.gov/35440408/

Jonathan Guo linkedin.com
Background: While the clinical benefits of CFTR modulators are clear, their high prices render them inaccessible outside of the world’s richest countries. Despite this, there is currently limited evidence regarding global access to these transformative therapies. Therefore, this study aims to estimate the minimum costs of production of CFTR modulators, assuming robust generic competition, and to compare them with current list prices to evaluate the feasibility of increased global access to treatment.
Methods: Minimum costs of production for CFTR modulators were estimated via an algorithm validated in previous literature and identification of cost-limiting key starting materials from published routes of chemical synthesis. This algorithm utilised per kilogram active pharmaceutical ingredient costs obtained from global import/export data. Estimated production costs were compared with published list prices in a range of countries.
Results: Costs of production for elexacaftor/tezacaftor/ivacaftor are estimated at $5,676 [$4,628-6,723] per year, over 90% lower than the US list price. Analysis of chemical structure and published synthetic pathways for elexacaftor/tezacaftor/ivacaftor revealed relatively straightforward routes of synthesis related to currently available products. Total cost of triple therapy for all eligible diagnosed CF patients worldwide would be $489 million per year. Comparatively, the annual cost at US list price would be $31.2 billion.
Conclusions: Elexacaftor/tezacaftor/ivacaftor could be produced via generic companies for a fraction of the list price. The current pricing model restricts access to the best available therapy, thereby exacerbating existing inequalities in CF care. Urgent action is needed to increase the availability of triple combination treatment worldwide. One strategy based on previous success is originator-issued voluntary licenses.
Caitlyn Harvey, Sinead Weldon, Stuart Elborn, Damian G Downey, Clifford Taggart. The Effect of CFTR Modulators on Airway Infection in Cystic Fibrosis. Int J Mol Sci 2022 Mar 23;23(7):3513.doi: 10.3390/ijms23073513. [Pubmed]

Caitlyn Harvey facebook.com
The advent of Cystic fibrosis transmembrane receptor (CFTR) modulators in 2012 was a critical event in the history of cystic fibrosis (CF) treatment. Unlike traditional therapies that target downstream effects of CFTR dysfunction, CFTR modulators aim to correct the underlying defect at the protein level. These genotype-specific therapies are now available for an increasing number of CF patients, transforming the way we view the condition from a life-limiting disease to one that can be effectively managed.
Several studies have demonstrated the vast improvement CFTR modulators have on normalization of sweat chloride, CFTR function, clinical endpoints, and frequency of pulmonary exacerbation. However, their impact on other aspects of the disease, such as pathogenic burden and airway infection, remain under explored. Frequent airway infections as a result of increased susceptibility and impaired innate immune response are a serious problem within CF, often leading to accelerated decline in lung function and disease progression.
Current evidence suggests that CFTR modulators are unable to eradicate pathogenic organisms in those with already established lung disease. However, this may not be the case for those with relatively low levels of disease progression and conserved microbial diversity, such as young patients. Furthermore, it remains unknown whether the restorative effects exerted by CFTR modulators extend to immune cells, such as phagocytes, which have the potential to modulate the response of people with CF (pwCF) to infection. Throughout this review, we look at the potential impact of CFTR modulators on airway infection in CF and their ability to shape impaired pulmonary defences to pathogens.
Dr Caitlyn Harvey is in the Airway Innate Immunity Research (AiiR) Group, Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK.
Eunjin Hong, Lisa M Almond, P S Chung, A Peter Rao, Paul M Beringer PBPK-led guidance for cystic fibrosis patients taking elexacaftor-tezacaftor-ivacaftor with nirmatrelvir-ritonavir for the treatment of COVID-19. Clin Pharmacol Ther 2022 Mar 16.doi: 10.1002/cpt.2585. Online ahead of print. [Pubmed]

Eunjin Hong
Cystic fibrosis transmembrane conductance regulator (CFTR) modulating therapies including elexacaftor, tezacaftor, and ivacaftor (ETI) are primarily eliminated through cytochrome P450 (CYP) 3A-mediated metabolism. This creates a therapeutic challenge to the treatment of COVID-19 with nirmatrelvir-ritonavir in people with cystic fibrosis (CF) due to the potential for significant drug-drug interactions (DDI). However, CF population is more at risk of serious illness following COVID-19 infection and hence it is important to manage the DDI risk and provide treatment options.
CYP3A-mediated DDI of ETI was evaluated using a physiologically based pharmacokinetic (PBPK) modeling approach. Modeling was performed incorporating physiological information and drug dependent parameters of ETI to predict the effect of ritonavir (the CYP3A inhibiting component of the combination) on pharmacokinetics of ETI. The ETI models were verified using independent clinical pharmacokinetic and DDI data of ETI with a range of CYP3A modulators. When ritonavir was administered on day 1 through 5, the predicted AUC ratio of ivacaftor (the most sensitive CYP3A substrate) on day 6 was 9.31, indicating that its metabolism was strongly inhibited.
Based on the predicted DDI, the dose of ETI should be reduced when co-administered with nirmatrelvir-ritonavir to elexacaftor 200mg-tezacaftor 100mg-ivacaftor 150mg on days 1 and 5, with delayed resumption of full dose ETI on day 9, considering the residual inhibitory effect of ritonavir as a mechanism-based inhibitor. The simulation predicts a regimen of ETI administered concomitantly with nirmatrelvir/ritonavir in people with CF that will likely decrease the impact of the drug interaction.
Eunjin Hong is a graduate student the Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, 1985 Zonal Ave, Los Angeles, CA, 90033, USA.
Brionna N Hudson, Hollyann R Jacobs , Alexander Philbrick, Xiaolong A Zhou, Michelle M Simonsen, Julie A Safirstein, Shannon M Rotolo. Drug-induced acne with elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis. J Cyst Fibros 2022 Sep 7;S1569-1993(22)00657-9.doi: 10.1016/j.jcf.2022.09.002.Online ahead of print. pubmed.ncbi.nlm.nih.gov/36088208/

Brianna S Hudson linkedin,com
Elexacaftor/tezacaftor/ivacaftor (ELX-TEZ-IVA) is a Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator shown to improve lung function and reduce sweat chloride in people with Cystic Fibrosis (CF). The only commonly reported dermatologic adverse effect with CFTR modulators including ELX-TEZ-IVA is rash. In this case series, we describe 19 patients who reported new onset or worsening of acne after initiation of this drug to their CF pharmacist or another member of their CF care team. The mechanism and frequency of this adverse effect is unknown.
Dr Brionna N Hudson is at the College of Pharmacy Chicago State University
Kazani S, Rowlands DJ, Bottoli I, Milojevic J, Alcantara J, Jones I, Kulmatycki K, Machineni S, Mostovy L, Nicholls I, Nick JA, Rowe SM, Simmonds NJ, Vegesna R, Verheijen J, Danahay H, Gosling M, Ayalavajjala PS, Salman M, Strieter R. Safety and efficacy of the cystic fibrosis transmembrane conductance regulator potentiator icenticaftor (QBW251). J Cyst Fibros. 20(2):250-256, 2021 03. Free article [Pubmed]
Background: This is the first-in-human study of icenticaftor, an oral potentiator of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) channel. Restoration of CFTR activity has shown significant clinical benefits, but more studies are needed to address all CFTR mutations.
Methods: Safety, pharmacodynamics/pharmacokinetics of icenticaftor were evaluated in a randomized, double-blind, placebo-controlled study in healthy volunteers. Efficacy was assessed in adult CF patients with ≥1 pre-specified CFTR Class III or IV mutation (150 and 450 mg bid), or homozygous for F508del mutation (450 mg bid). Primary efficacy endpoint was change from baseline in lung clearance index (LCI2.5). Secondary endpoints included %predicted FEV1 and sweat chloride level.
Results: Class IV mutations were present in 22 patients, Class III in 2 (both S549N), and 25 were homozygous for F508del. Icenticaftor was well-tolerated in healthy and CF subjects with no unexpected events or discontinuations in the CF groups. The most frequent study-drug related adverse events in CF patients were nausea (12.2%), headache (10.2%), and fatigue (6.1%). Icenticaftor 450 mg bid for 14 days showed significant improvements in all endpoints versus placebo in patients with Class III and IV mutations; mean %predicted FEV1 increased by 6.46%, LCI2.5 decreased by 1.13 points and sweat chloride decreased by 8.36 mmol/L. No significant efficacy was observed in patients homozygous for a single F508del.
Conclusions: Icenticaftor was safe and well-tolerated in healthy volunteers and CF patients, and demonstrated clinically meaningful changes in lung function and sweat chloride level in CF patients with Class III and IV CFTR mutations. (ClinicalTrials.gov: NCT02190604).
Dr Shamsah Kazani is at Novartis Institutes for BioMedical Research, Cambridge, MA, United States; Novartis Institutes for BioMedical Research, Basel, Switzerland.
Rebecca Keyte, Sophia Kauser, Michail Mantzios, Helen Egan. The psychological implications and health risks of cystic fibrosis pre- and post- CFTR modulator therapy. Chronic Illn 2022 May 3;17423953221099042.doi: 10.1177/17423953221099042.Online ahead of print.[Pubmed]

Rebecca Keyte bcu.ac.uk
Objectives: Cystic Fibrosis (CF) care is entering a period of personalised medicine with the emergence of CF transmembrane conductance regulator (CFTR) modulator therapies. Anecdotally individuals are reporting life-changing effects of modulator therapies, proposing an important area of study.
Methods: Twenty adult participants (males: 8, age range: 22-51 years, average FEV1: 53.45%) were recruited via social media to participate in a semi-structured interview; 17 participants were currently taking Elexacaftor/Tezacaftor/Ivacaftor (Kaftrio).
Results: An appreciation of a “normal life” post-modulator therapy is paramount, with improvements in symptoms and quality-of-life bringing a more urgent imperative for the provision of effective support to encourage positive health and lifestyle choices.
Discussion: In this new era of CF care, there remains many challenges present for the CF community, with participants suggesting that proactive psychological support is required along with proactive awareness regarding health risk behaviours for the current and future CF generations.
Rebecca Keyte is in the Department of Psychology, School of Social Sciences, 34651 Birmingham City University, Birmingham, UK.
Insa Korten, Elisabeth Kieninger, Linn Krueger, Marina Bullo, Christa E Flück, Philipp Latzin, Carmen Casaulta, Claudia Boettcher Short-Term Effects of Elexacaftor/Tezacaftor/Ivacaftor Combination on Glucose Tolerance in Young People With Cystic Fibrosis-An Observational Pilot Study. Front Pediatr 2022 Apr 21;10:852551.doi: 10.3389/fped.2022.852551. eCollection 2022. [Pubmed]

Insa Korten kinderklinik.insel.ch
The effect of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) on glucose tolerance and/or cystic-fibrosis-related diabetes (CFRD) is not well understood. We performed an observational study on the short-term effects of ELX/TEZ/IVA on glucose tolerance.
Methods: Sixteen adolescents with CF performed oral glucose tolerance tests (OGTT) before and 4-6 weeks after initiating ELX/TEZ/IVA therapy. A continuous glucose monitoring (CGM) system was used 3 days before until 7 days after starting ELX/TEZ/IVA treatment.
Results: OGTT categories improved after initiating ELX/TEZ/IVA therapy (p = 0.02). Glucose levels of OGTT improved at 60, 90, and 120 min (p < 0.05), whereas fasting glucose and CGM measures did not change.
Conclusion: Shortly after initiating ELX/TEZ/IVA therapy, glucose tolerance measured by OGTT improved in people with CF. This pilot study indicates that ELX/TEZ/IVA treatment has beneficial effects on the endocrine pancreatic function and might prevent or at least postpone future CFRD.
Insa Korten is in the Division of Paediatric Respiratory Medicine and Allergology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Benjamin C Lyman, Jessica Seay, Casey Contreary, Adrienne P Savant, Mary Lynn Dell, George C Hescock. lexacaftor-tezacaftor-ivacaftor overdose in an adolescent female with cystic fibrosis. Pediatr Pulmonol 2022 Aug 12. doi: 10.1002/ppul.26116. Online ahead of print.[Pubmed]

Benjamin C Lyman icmchealth.org
Summary of the letter to the Editor A 15-year-old female with CF (621+1G>T and F508del), pancreatic insufficiency, CF-related diabetes requiring insulin, and recurrent major depressive and anxiety disor e ders was started on ETI approximately 10 months before an intentional ingestion of an estimated 42 pills of ETI (two 1-week blister packs) in a suicide attempt. Less than 1 h post-ingestion, she self-induced emesis, then presented to the emergency department (ED), where she also complained of increased sputum production consistent with a pulmonary exacerbation. Vital signs and physical exam were within normal limits for age. Complete blood count, comprehensive metabolic panel (CMP), coagulation markers, urine drug screen, salicylate, acetaminophen, ethanol, and electrocardiogram were within normal limits.
Maximum elevation in LFTs was seen at 12-day postingestion and noted to be 5.7 and 4.1 times the upper limit of normal for AST and ALT, respectively. Given the primary concern of liver damage with ETI toxicity, the patient’s ETI dose was held for 9 days. On day 10 postingestion, following LFT reduction to less than twice the upper limit of normal, the two pill, morning dose (ETI: 100-50-75 mg) was restarted. Her LFTs had an acute rise on day 12 postingestion; therefore, ETI was held for an additional day. On day 14 postingestion, the morning dose was again restarted. LFTs returned to normal range by day 19 postingestion. The nighttime dose (ivacaftor 150 mg) was restarted on day 28 postingestion, after sustained stabilization of LFTs..
In conclusion, as care for pwCF enters the modulator era, it is critical to partner with mental health providers, screen pwCF for mental health issues, and recognize the potential for worsening of mental health with modulators.
Dr Benjamin C Lyman is a paediatrician at the Children’s Hospital New Orleans
Teresa Fuchs, Dorothea Appelt, Katharina Niedermayr, Helmut Ellemunter. REAL-world clinical effectiveness of ivacaftor therapy in the first 24 months in two infants with cystic fibrosis and different gating mutations-A case report. Clin Case Rep 2022 Feb 10;10(2):e05364.doi: 10.1002/ccr3.5364.eCollection 2022 Feb Free PMC article [Pubmed]
This study summarizes efficacy of ivacaftor treatment in 2 infants in a real-world setting. Two infants aged 2 and 11 months were started on ivacaftor off label with parental consent. A distinct decline of sweat chloride and lung clearance index plus increase in fecal elastase was seen. No side effects occurred. The results underline the early and sustainable effect in patients started aged less than 12 months and give cause for discussing whether a reduction in standard cystic fibrosis therapy is possible. Further details in the Free PMC article where other studies on young infants are discussed.
Teresa Fuchs is at the Medical University of Innsbruck Cystic Fibrosis Centre Innsbruck Innsbruck Austria
Brittany Miles, Jay Chacko, Mohammed Zaidan. The Impact of Elexacaftor/Ivacaftor/Tezacaftor on Cystic Fibrosis Patients Who Acquire COVID-19 Infection Cureus. 2022 Sep 17;14(9):e29276. doi: 10.7759/cureus.29276. eCollection 2022 Sep pubmed.ncbi.nlm.nih.gov/36277555
The combination of medication containing elexacaftor, ivacaftor, and tezacaftor (EIT) has dramatically impacted the treatment and prognosis for patients with cystic fibrosis (CF). Lung function, weight, and self-reported quality of life have improved for many of these patients, but little is known about whether this treatment will have a beneficial effect in preventing morbidity and/or mortality from respiratory infections such as COVID-19. EIT received Food and Drug Administration (FDA) approval shortly before the first cases of COVID-19 appeared in the United States. We performed an analysis using the TriNetX (Cambridge, MA, USA) research database to determine if patients being treated with EIT who became infected with COVID-19 experienced significantly different outcomes compared to patients who were not receiving it.
Brittany Miles is at Medical Education and Radiology, University of Texas Medical Branch, Galveston, USA.
Sarah Jane Schwarzenberg , Phuong T Vu, Michelle Skalland , Lucas R Hoffman, Christopher Pope, Daniel Gelfond, et al and the Promise Study Group. Elexacaftor/tezacaftor/ivacaftor and gastrointestinal outcomes in cystic fibrosis: Report of promise-GI. J Cyst Fibros
. 2022 Oct 21;S1569-1993(22)01384-4. doi: 10.1016/j.jcf.2022.10.003. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36280527/

Sarah Jane Schwarzenberg
med.umn.edu
Background: Elexacaftor/tezacaftor/ivacaftor (ETI) improves pulmonary disease in people with cystic fibrosis (PwCF), but its effect on gastrointestinal symptoms, which also affect quality of life, is not clear.
Methods: PROMISE is a 56-center prospective, observational study of ETI in PwCF >12 years and at least one F508del allele. Gastrointestinal symptoms, evaluated by validated questionnaires: Patient Assessment of Upper Gastrointestinal Disorders-Symptom (PAGI-SYM), Patient Assessment of Constipation-Symptom (PAC-SYM), Patient Assessment of Constipation-Quality of Life (PAC-QOL)), fecal calprotectin, steatocrit and elastase-1 were measured before and 6 months after ETI initiation. Mean difference and 95% confidence intervals were obtained from linear regression with adjustment for age and sex.
Results: 438 participants fully completed at least 1 questionnaire. Mean (SD) for baseline PAGI-SYM, PAC-SYM, and PAC-QOL total scores were 0.56 (0.59), 0.47 (0.45), and 0.69 (0.53) out of maximum 5, 4, and 5, respectively (higher score indicates greater severity). Corresponding age- and sex-adjusted 6 months mean changes (95% CI) in total scores were -0.15 (-0.21, -0.09) for PAGI-SYM, -0.14 (-0.19, -0.09) for PAC-SYM, and -0.15 (-0.21, -0.10) for PAC-QOL. While statistically significant, changes were small and unlikely to be of clinical importance. Fecal calprotectin showed a change (95% CI) from baseline of -66.2 µg/g (-86.1, -46.2) at 6 months, while fecal elastase and steatocrit did not meaningfully change.
Conclusions: After 6 months of ETI, fecal markers of inflammation decreased. Gastrointestinal symptoms improved, but the effect size was small. Pancreatic insufficiency did not improve
Professor Sarah Jane Schwarzenberg at Pediatrics, University of Minnesota Masonic Children’s Hospital, Academic Office Building, 2450 Riverside Ave S AO-201, Minneapolis, MN 55454, USA.
Jennifer L Goralski , Sang Hun Chung , Agathe S Ceppe , Margret Z Powell, Muthu Sakthivel, Brian D Handly, Yueh Z Lee , Scott H Donaldson.Dynamic Perfluorinated Gas MRI Shows Improved Lung Ventilation in People with Cystic Fibrosis after Elexacaftor/Tezacaftor/Ivacaftor: An Observational Study. J Clin Med. 2022 Oct 19;11(20):6160. doi: 10.3390/jcm11206160. pubmed.ncbi.nlm.nih.gov/36294480/

Jennifer Goralski
UNC School of medicine
The availability of highly effective CFTR modulators is revolutionizing the treatment of cystic fibrosis (CF) and drastically improving outcomes. MRI-based imaging modalities are now emerging as highly sensitive endpoints, particularly in the setting of mild lung disease. Adult CF patients were recruited from a single center prior to starting treatment with E/T/I. The following studies were obtained before and after one month on treatment: spirometry, multiple breath nitrogen washout (MBW), 1H UTE MRI (structural images) and 19F MRI (ventilation images). Changes between visits were calculated, as were correlations between FEV1, lung clearance index (LCI), MRI structural scores, and MRI-based ventilation descriptors. Eight subjects had complete datasets for evaluation. Consistent with prior clinical trials, FEV1 and LCI improved after 28 days of E/T/I use. 1H UTE MRI detected improvements in bronchiectasis/airway wall thickening score and mucus plugging score after 28 days of therapy. 19F MRI demonstrated improvements in fractional lung volume with slow gas washout time (FLV↑tau2) and ventilation defect percentage (VDP). Improvements in FLV↑tau2 and VDP correlated with improvement in FEV1 (r = 0.81 and 0.86, respectively, p < 0.05). This observational study establishes the ability of 19F MRI and 1H UTE MRI to detect improvements in lung structure and function after E/T/I treatment. This study supports further development of 19F MRI and 1H UTE MRI as outcome measures for cystic fibrosis research and drug development.
Dr Jennifer Goralski is at Division of Pulmonary and Critical Care Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, ; the Marsico Lung Institute/UNC Cystic Fibrosis Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, and the Division of Pediatric Pulmonology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Nicole Mayer-Hamblett , Felix Ratjen , Renee Russell , Scott H Donaldson , Kristin A Riekert , Gregory S Sawicki , Katherine Odem-Davis , Julia K Young , Daniel Rosenbluth , Jennifer L Taylor-Cousar , Christopher H Goss , George Retsch-Bogart , John Paul Clancy , Alan Genatossio , Brian P O’Sullivan , Ariel Berlinski , Susan L Millard , Gregory Omlor , Colby A Wyatt, Kathryn Moffett , David P Nichols , Alex H Gifford 20 , SIMPLIFY Study Group.Discontinuation versus continuation of hypertonic saline or dornase alfa in modulator treated people with cystic fibrosis (SIMPLIFY): results from two parallel, multicentre, open-label, randomised, controlled, non-inferiority trial. Lancet Respir Med. 2022 Nov 4;S2213-2600(22)00434-9. doi: 10.1016/S2213-2600(22)00434-9. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36343646/

Nicole Mayer-Hamblett
Background: Reducing treatment burden is a priority for people with cystic fibrosis, whose health has benefited from using new modulators that substantially increase CFTR protein function. The SIMPLIFY study aimed to assess the effects of discontinuing nebulised hypertonic saline or dornase alfa in individuals using the CFTR modulator elexacaftor plus tezacaftor plus ivacaftor (ETI).
Methods: The SIMPLIFY study included two parallel, multicentre, open-label, randomised, controlled, non-inferiority trials at 80 participating clinics across the USA in the Cystic Fibrosis Therapeutics Development Network. We included individuals with cystic fibrosis aged 12-17 years with percent predicted FEV1 (ppFEV1) of 70% or more, or those aged 18 years or older with ppFEV1 of 60% or more, if they had been taking ETI and either (or both) mucoactive therapies (≥3% hypertonic saline or dornase alfa) for at least 90 days before screening. Participants on both hypertonic saline and dornase alfa were randomly assigned to one of the two trials, and those on a single therapy were assigned to the applicable trial. All participants were then randomly assigned 1:1 to continue or discontinue therapy for 6 weeks using permuted blocks of varying size, stratified by baseline ppFEV1 (week 0; ≥90% or <90%), single or concurrent use of hypertonic saline and dornase alfa, previous SIMPLIFY study participation (yes or no), and age (≥18 or <18 years). For participants randomly assigned to continue their therapy during a given trial, this therapy was instructed to be taken at least once daily according to each participant’s pre-existing, clinically prescribed regimen. Hypertonic saline concentration was required to be at least 3%. The primary objective for each trial was to determine whether discontinuing was non-inferior to continuing, measured by the 6-week change in ppFEV1 in the per-protocol population. We established a non-inferiority margin of -3% for the difference between groups in the 6-week change in ppFEV1. Safety outcomes were analysed in the intention-to-treat population. This study is registered with ClinicalTrials.gov, NCT04378153.
Findings: From Aug 25, 2020, to May 25, 2022, a total of 672 unique participants were screened for eligibility for one or both trials, resulting in 847 total random assignments across both trials with 594 unique participants. 370 participants were randomly assigned in the hypertonic saline trial and 477 in the dornase alfa trial. Participants across both trials had an average ppFEV1 of 96·9%. Discontinuing treatment was non-inferior to continuing treatment with respect to the absolute 6-week change in ppFEV1 in both the hypertonic saline trial (-0·19% [95% CI -0·85 to 0·48] in the discontinuation group [n=133] vs 0·14% [-0·51 to 0·78] in the continuation group [n=140]; between-group difference -0·32% [-1·25 to 0·60]) and dornase alfa trial (0·18% [-0·38 to 0·74] in the discontinuation group [n=199] vs -0·16% [-0·73 to 0·41] in the continuation group [n=193]; between-group difference 0·35% [-0·45 to 1·14]), with consistent results in the intention-to-treat populations. In the hypertonic saline trial, 64 (35%) of 184 in the discontinuation group versus 44 (24%) of 186 participants in the continuation group and, in the dornase alfa trial, 89 (37%) of 240 in the discontinuation group versus 55 (23%) of 237 in the continuation group had at least one adverse event.
Interpretation: In individuals with cystic fibrosis on ETI with relatively well preserved pulmonary function, discontinuing daily hypertonic saline or dornase alfa for 6 weeks did not result in clinically meaningful differences in pulmonary function when compared with continuing treatment.
Nicole Mayer-Hamblett is at the Children’s Research Institute, Seattle, WA, USA; Department of Pediatrics, University of Washington, Seattle, WA, USA; Department of BiostatisticUniversity of Washington Seattle USA
Stefanie Vincken , Sylvia Verbanck , Sue Braun , Nathalie Buyck , Christiane Knoop , Eef Vanderhelst. Real-world data on the efficacy and safety of tezacaftor-ivacaftor in adults living with cystic fibrosis homozygous for F508del and heterozygous for F508del and a residual function mutation. Acta Clin Belg
. 2022 Nov 22;1-5. doi: 10.1080/17843286.2022.2145684. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36415912/
Background: To examine safety and efficacy of tezacaftor-ivacaftor (TEZ/IVA) in a real-life setting in adults living with cystic fibrosis.
Methods: A multicentre retrospective observational study, including adults living with cystic fibrosis (pwCF) eligible for TEZ/IVA, with assessments at baseline, 3 months (visit3mo) and 6 months (visit6mo) after start of treatment. Outcomes included change in FEV1, LCI, FeNO, CFQ-R, estimated number of annual acute exacerbations, BMI, dosage of pancreatic enzyme replacement therapy (PERT) and airway microbiology. We also assessed safety.
Results: Forty-eight adult pwCF (mean (±SD) age 33 (±12) years; mean FEV1 65 (±19) %P) were included. Three subgroups were identified: pwCF F/F CFTR modulator-naive (n = 28; 58%), pwCF F/F previously treated with lumacaftor-ivacaftor (n = 11; 23%) and pwCF F/RF (n = 9; 19%). Adverse events were described in 3 pwCF (6%) during the 6-month observation period (in one leading to treatment interruption). At visit3mo, FEV1 had improved in all subgroups. In the entire group, mean FEV1 had increased from 66 (±2.9) %P to 72 (±2.9) %P (p < 0.0001). Similarly, LCI improved by approximately one unit at visit3mo (p = 0.02). At visit6mo mean annual acute exacerbation rate decreased significantly (p = 0.02). Only in the CFQ-R social functioning domain score, a significant improvement was observed at visit6mo (p < 0.01).
Conclusions: We showed that TEZ/IVA (Symdeko) is safe, well tolerated and effective in terms of improvement of lung function, ventilation inhomogeneity, health-related social functioning, and reduction of estimated annual acute exacerbation rate, in adult pwCF F/F and F/RF. Results in this real-life study reflect those observed in RCTs
Stefanie Vincken in is the Department of Pulmonology, Vrije Universiteit Brussel (VUB), Universitair Ziekenhuis Brussel (UZ Brussel), Brussels, Belgium.
Meghana Sathe , Baha Moshiree , Enid Aliaj , MinJae Lee , Jessica Hudson , Alex Gifford , Susan Attel , Breck Gamel Med , Steven D Freedman , Sarah Jane Schwarzenberg , A Jay Freeman. Need to study simplification of gastrointestinal medication regimen in cystic fibrosis in the era of highly effective modulators.Pediatr Pulmonol. 2022 Nov 30. doi: 10.1002/ppul.26257. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36448312/

Meghana Nitin Sathe
Introduction: The success of highly effective modulator therapy (HEMT) has led to consideration of simpler regimens for people with CF (PwCF) with opportunities to modify burdensome regimens. Despite the intuitive appeal of discontinuing chronic therapies no longer necessary, this process should be pursued systematically to ensure safety, adherence, and validate patient-centered preferences. We designed a questionnaire to determine the state of use of acid suppressive medications (ASM) and pancreatic enzyme therapy (PERT), current self-withdrawal and provider-directed withdrawal practices, and interest in a standardized withdrawal study.
Methods: In collaboration with CF Foundation (CFF), a questionnaire was developed and distributed to members of Community Voice (CV, comprised of PwCF and their loved ones), and CF providers regarding the need to study simplifying the gastrointestinal (GI) regimen for PwCF on HEMT.
Results: Approximately 20-40% of CV or CF providers have decreased or stopped ASM for those on HEMT. For PERT, CV and CF providers have decreased dose (34-48% and approximately 25%, respectively) more often than having stopped it altogether (13-24% and 3-12%, respectively). Cumulatively, there is interest in pursuing research in this area (86% CV, 89% CF providers) and willingness to enroll in such a study (80% CV and 89% CF providers).
Conclusion: Systematically studying withdrawal of common GI medications, ASM and PERT, is important to CV and CF providers. Decreases in dosing and withdrawal are already taking place without evidence to support this practice. This questionnaire is the first step in designing a GI medication simplification study in PwCF on HEMT. This article is protected by copyright. All rights reserved.
Dr Meghana Sathe is a paedaitric gastroenterologist and professor at Pediatric Gastroenterology, Hepatology and Nutrition, University of Texas Southwestern/Children’s Health, Dallas, TX.
Shahid Sheikh, Rodney D Britt Jr, Nancy A Ryan-Wenger, Aiman Q Khan, Brandon W Lewis, Courtney Gushue, Hazel Ozuna, Devi Jaganathan, Karen McCoy, Benjamin T Kopp. Impact of Elexacaftor-Tezacaftor-Ivacaftor on Bacterial Colonization and Inflammatory Responses in Cystic Fibrosis. Pediatr Pulmonol. 2022 Nov 29. doi: 10.1002/ppul.26261. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36444736/

Shahid Sheikh
Background: Cystic fibrosis (CF) is a multisystem disease with progressive deterioration. Recently, CF transmembrane conductance regulator (CFTR) modulator therapies were introduced that repair underlying protein defects. Objective of this study was to determine the impact of elexacaftor-tezacaftor-ivacaftor (ETI) on clinical parameters and inflammatory responses in people with CF (pwCF). Methods: Lung function (FEV1 ), body mass index (BMI) and microbiologic data were collected at initiation and 3-month intervals for 1 year. Blood was analyzed at baseline and 6 months for cytokines and immune cell populations via flow cytometry and compared to non-CF controls.
Results: Sample size was 48 pwCF, 28 (58.3%) males with a mean age of 28.8±10.7 years. Significant increases in %predicted FEV1 and BMI were observed through 6 months of ETI therapy with no change thereafter. Changes in FEV1 and BMI at 3 months were significantly correlated (r=57.2, p<0.01). There were significant reductions in Pseudomonas and Staphylococcus positivity (percent of total samples) in pwCF through 12 months of ETI treatment. Healthy controls (n=20) had significantly lower levels of circulating neutrophils, IL-6, IL-8, and IL-17A and higher levels of IL-13 compared to pwCF at baseline (n=48). After 6 months of ETI, pwCF had significant decreases in IL-8, IL-6, and IL-17A levels and normalization of peripheral blood immune cell composition.
Conclusions: In pwCF, ETI significantly improved clinical outcomes, reduced systemic pro-inflammatory cytokines, and restored circulating immune cell composition after 6 months of therapy.
Dr Shahid Sheikh is a paediatric pulmonologist in the Department of Pediatrics, The Ohio State University College of Medicine, Columbus, Ohio, and the Division of Pulmonary Medicine, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Renate Kos , Anne H Neerincx, Dominic W Fenn , Paul Brinkman , Rianne Lub , Steffie E M Vonk , Jolt Roukema, Monique H Reijers, Suzanne W J Terheggen-Lagro, Josje Altenburg Christof J Majoor, Lieuwe D Bos, Eric G Haarman , Anke H Maitland-van der Zee, Amsterdam Mucociliary Clearance Disease (AMCD) Research Group.. Real-life efficacy and safety of elexacaftor/tezacaftor/ivacaftor on severe cystic fibrosis lung disease patients. Observational Study Pharmacol Res Perspect. 2022 Dec;10(6):e01015. doi: 10.1002/prp2.1015. Free article pubmed.ncbi.nlm.nih.gov/36440690/

Renate Kos
Elexacaftor/tezacaftor/ivacaftor (ETI) is a cystic fibrosis (CF) transmembrane conductance regulator modulator, which has shown efficacy in CF patients (≥6 years) with ≥1 Phe508del mutation and a minimal function mutation. In October 2019, ETI became available on compassionate use basis for Dutch CF patients with severe lung disease. Our objective was to investigate safety and efficacy of ETI in this patient group in a real-life setting. A multicenter longitudinal observational study was conducted to examine changes in FEV1 , BMI, and adverse events at initiation and 1, 3, 6, and 12 months after starting ETI. The number of exacerbations was recorded in the 12 months before and the 12 months after ETI treatment. Patients eligible for compassionate use had a FEV1 <40% predicted. Wilcoxon signed-rank test analyzed changes over time. Twenty subjects were included and followed up for up to 12 months after starting ETI. Treatment was well tolerated with mild side effects reported, namely, rash (15%) and stomach ache (20%) with 80% resolving within 1 month. Mean absolute increase of FEV1 was 11.8/13.7% (p ≤ .001) and BMI was 0.49/1.87 kg/m2 (p < .001-0.02) after 1/12 months, respectively. In comparison to the number of exacerbations pretrial, there was a marked reduction in exacerbations after initiation. Our findings show long-term effects of treatment with ETI in patients with severe CF lung disease in a real-life setting. Treatment with ETI is associated with increased lung function and BMI, less exacerbations, and only mild side effects.
Renate Kos is a PhD student in the Department of Respiratory Medicine, Amsterdam University Medical Centres – loc. AMC, Amsterdam, The Netherlands.
Lakshmi Viswanathan, Eric Bachman, Simon Tian, Neil Ahluwalia, Yaohua Zhang, Harold S Bernstein, Paul Panorchan.Phase 1 Study to Assess the Safety and Pharmacokinetics of Elexacaftor/Tezacaftor/Ivacaftor in Subjects Without Cystic Fibrosis With Moderate Hepatic Impairment. Eur J Drug Metab Pharmacokinet 2022 Aug 29.doi: 10.1007/s13318-022-00791-8.Online ahead of print pubmed.ncbi.nlm.nih.gov/36036885/
Background and objective:Elexacaftor/tezacaftor/ivacaftor is highly effective in treating people with cystic fibrosis (pwCF) who have ≥ 1 responsive mutation. Liver disease occurs in approximately 10%-20% of pwCF. The objective of this study was to assess the safety and pharmacokinetics of elexacaftor/tezacaftor/ivacaftor in people with moderate hepatic impairment, which is necessary to inform on its use and guide dosing recommendations.
Methods:The safety and pharmacokinetics of elexacaftor/tezacaftor/ivacaftor were evaluated in subjects without CF with moderate hepatic impairment versus matched healthy controls. Twenty-two subjects (11 with moderate hepatic impairment and 11 healthy subjects) received half the standard adult daily dose of elexacaftor/tezacaftor/ivacaftor (elexacaftor 100 mg/tezacaftor 50 mg/ivacaftor 150 mg) orally for 10 days.
Results:Elexacaftor/tezacaftor/ivacaftor was safe and well tolerated in subjects with moderate hepatic impairment and healthy controls. On day 10, the mean values of the area under the curve during the dosing interval (AUCτ) for total (bound and unbound) elexacaftor and its major active metabolite M23-elexacaftor were increased 1.25-fold (95% CI 1.01, 1.54) and 1.73-fold (95% CI 1.27, 2.35), respectively, in subjects with moderate hepatic impairment compared with matched healthy subjects. The mean values of AUCτ for ivacaftor and tezacaftor were increased 1.50-fold (95% CI 1.09, 2.06) and 1.20-fold (95% CI 1.00, 1.43), respectively, while the mean value of AUCτfor the active metabolite M1-tezacaftor was 1.29-fold lower [ratio of moderate hepatic impairment to healthy subjects (95% CI): 0.778 (0.655, 0.924)] in subjects with moderate hepatic impairment.
Conclusions:A dose reduction of elexacaftor/tezacaftor/ivacaftor is warranted in people with moderate hepatic impairment.
Lakshmi Viswanathan is a clinical pharmacologist with Vertex Phamaceuticals in Boston USA
-I wonder if the non-CF patients were expected to improve. Were they informed it was not for their benefit? This paper is available in full on publisher’s website
Julie Mésinèle, Manon Ruffin, Loïc Guillot, Pierre-Yves Boëlle, Harriet Corvol, On Behalf Of The French Cf Modifier Gene Study Investigators. Factors predisposing the response to Lumacaftor/ivacaftor in people with cystic fibrosis. J Pers Med 2022 Feb 10;12(2):252.doi: 10.3390/jpm12020252. Free PMC articlepubmed.ncbi.nlm.nih.gov/35207740

Julie Mesinele
Lumacaftor/ivacaftor (LUMA-IVA) therapy is prescribed to people with cystic fibrosis (pwCF) homozygous for the Phe508del-CFTR variant to restore CFTR protein function. There is, however, large inter-individual variability in treatment response. Here, we seek to identify clinical and/or genetic factors that may modulate the response to this CFTR modulator therapy.
A total of 765 pwCF older than 12 years under LUMA-IVA therapy and with lung function and nutritional measurements available before and after treatment initiation were included. Response to treatment was determined by the change in lung function and nutritional status, from baseline and over the first two years after initiation, and it was assessed by weighted generalized estimating equation models. Gains in lung function and nutritional status were observed after 6 months of treatment (on average 2.11 ± 7.81% for percent predicted FEV1 and 0.44 ± 0.77 kg/m2 for BMI) and sustained over the 2 years.
We observed that the more severe patients gained the most in lung function and nutritional status. While females started with a nutritional status more impaired than males, they had a larger response and regained BMI Z-score values similar to men after 2 years of treatment. We observed no association between variants in solute carrier (SLC) genes and the respiratory function response to LUMA-IVA, but the SLC6A14 rs12839137 variant was associated with the nutritional response. Further investigations, including other genomic regions, will be needed to fully explore the inter-individual variability of the response to LUMA-IVA.
Dr Julie Mésinèle is Post-Doctoral Researcher in Biostatistics at the Centre de Recherche Saint-Antoine (CRSA), Inserm, Sorbonne Université, 75012 Paris, France. and Hôpital Saint-Antoine, AP-HP, Institut Pierre Louis d’Epidémiologie et de Santé Publique (iPLESP), Inserm, Sorbonne Université, 75012 Paris, France
Lena Wucherpfennig, Simon M F Triphan, Sabine Wege, Hans-Ulrich Kauczor, Claus P Heussel, Niclas Schmitt, Felix Wuennemann, Victoria L Mayer, Olaf Sommerburg, Marcus A Mall, Monika Eichinger, Mark O Wielpütz Magnetic resonance imaging detects improvements of pulmonary and paranasal sinus abnormalities in response to elexacaftor/tezacaftor/ivacaftor therapy in adults with cystic fibrosis. J Cyst Fibros 2022 Apr 7;S1569-1993(22)00088-1.: 10.1016/j.jcf.2022.03.011.Online ahead of print.[Pubmed]

Lena Wucherpfennig
researchgate.net
Background: Therapy with Elexacaftor/Tezacaftor/Ivacaftor (ETI) was recently approved for adult cystic fibrosis (CF) patients with at least one F508del mutation. However, its effects on structural and functional lung abnormalities and chronic rhinosinusitis have not been studied by imaging.
Methods: 19 adults with CF (mean age 31±9y, range 19-55y) underwent standardized chest magnetic resonance imaging (MRI), and nine also same-session sinonasal MRI, before (MRI1) and after (MRI2) at least one month (mean duration 5 ± 3mon) on ETI. 24 control CF patients (30±7y, range 20-44y) without ETI underwent longitudinal chest MRI, and eleven also sinonasal MRI, twice (mean interval 40±15mon). MRI was assessed using the validated chest MRI score and chronic rhinosinusitis (CRS)-MRI score. Forced expiratory volume in 1 s percent predicted (FEV1%) was measured in all patients.
Results: In controls, the chest MRI global score and CRS-MRI sum score were stable from MRI1 to MRI2. In patients under ETI, the chest MRI global score improved (-11.4 ± 4.6, P<0.001), mainly due to reduction of bronchiectasis/wall thickening and mucus plugging subscores (-3.3 ± 2.2 and -5.2 ± 1.5, P<0.001, respectively). The improvement in chest MRI score correlated well with improved FEV1% (r=-0.703, P<0.001). The CRS-MRI sum score also improved in patients under ETI (-6.9 ± 3.0, P<0.001), mainly due to a reduction of mucopyoceles in the maxillary and ethmoid sinus (-50% and -39%, P<0.05, respectively).
Conclusions: MRI detects improvements of chest MRI and CRS-MRI scores in adult CF patients who first received ETI, demonstrating reversibility of structural lung and paranasal sinus abnormalities in patients with established disease.
Dr Lena Wucherpfennig is in the Department of Diagnostic and Interventional Radiology, Subdivision of Pulmonary Imaging, University Hospital Heidelberg, Im Neuenheimer Feld 420, 69120 Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC), German Center for Lung Research (DZL), Im Neuenheimer Feld 156, 69120 Heidelberg, Germany; Department of Diagnostic and Interventional Radiology with Nuclear Medicine, Thoraxklinik at University Hospital Heidelberg, Röntgenstr. 1, 69126 Heidelberg, Germany.
Thomas S FitzMaurice , Caroline McCann, Dilip Nazareth , Matthew Shaw , Paul S McNamara , Martin J Walshaw Measuring the effect of elexacaftor/tezacaftor/ivacaftor combination therapy on the respiratory pump in people with CF using dynamic chest radiography. J Cyst Fibros. 2022 Nov;21(6):1036-1041. doi: 10.1016/j.jcf.2022.01.007. Epub 2022 Jan 31. 35101365 Free article pubmed.ncbi.nlm.nih.gov/35101365/

Thomas A Fitzmaurice
ResearchGate
Background: The CFTR modulator elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) leads to significant improvement in the symptoms and spirometry of people with cystic fibrosis (pwCF), but little evidence exists to understand its effect on respiratory pump function. Dynamic chest radiography (DCR) is a novel cineradiographic tool that identifies and tracks the chest wall and diaphragm throughout the breathing cycle, alongside fluoroscopic images of the chest of diagnostic quality. Methods: In this observational work, we examined the spirometry and DCR of 24 pwCF before and after starting ELX/TEZ/IVA. DCR automatically tracked the hemidiaphragm midpoints and projected lung area (PLA) during tidal and deep breathing manoeuvres.
Results: ppFEV1 (61±18 to 73±22, P<0.001) and ppFVC (77±16 to 88±15, P<0.001) improved significantly. DCR demonstrated a significant increase in hemidiaphragm excursion on both the right (18±11 to 26±9 mm, P<0.001) and left (21±11 to 31±11 mm, P<0.001) sides, as well as maximum hemidiaphragm speed during inspiration (right 22±14 to 31±11 mm/s, P=0.03; left 28±11 to 37±16 mm/s, P=0.02). PLA at end-expiration was significantly reduced (334±71 to 290±72cm2, P<0.001), with a significant increase in ΔPLA (83±40 to 117±36cm2, P<0.001).
Conclusions: DCR demonstrated significant improvements in hemidiaphragm excursion and ΔPLA in pwCF started on ELX/TEZ/IVA. These changes likely reflect a reduction in air trapping and improved elastic recoil of the chest, and are consistent with improvements seen in spirometry. The changes seen with DCR are physiologically plausible and correlate well with spirometry. DCR warrants further investigation as a tool for assessing the impact of CFTR-modulating therapies.
Thomas S FitzMaurice is at the Adult CF Unit, Liverpool Heart and Chest Hospital, Thomas Drive, Liverpool L14 3PE, UK; Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK.
Tamara Hillenaar, Jeffrey Beekman, Peter van der Sluijs, Ineke Braakman. Redefining Hypo- and Hyper-Responding Phenotypes of CFTR Mutants for Understanding and Therapy. Int J Mol Sci. 2022 Dec 2;23(23):15170. doi: 10.3390/ijms232315170. Free PMC article pubmed.ncbi.nlm.nih.gov/36499495/

Tamara Hillenaar
Mutations in CFTR cause misfolding and decreased or absent ion-channel function, resulting in the disease Cystic Fibrosis. Fortunately, a triple-modulator combination therapy (Trikafta) has been FDA-approved for 178 mutations, including all patients who have F508del on one allele. That so many CFTR mutants respond well to modulators developed for a single mutation is due to the nature of the folding process of this multidomain protein. We have addressed the question ‘What characterizes the exceptions: the mutants that functionally respond either not or extremely well’. A functional response is the product of the number of CFTR molecules on the cell surface, open probability, and conductivity of the CFTR chloride channel. By combining biosynthetic radiolabeling with protease-susceptibility assays, we have followed CF-causing mutants during the early and late stages of folding in the presence and absence of modulators. Most CFTR mutants showed typical biochemical responses for each modulator, such as a TMD1 conformational change or an increase in (cell-surface) stability, regardless of a functional response. These modulators thus should still be considered for hypo-responder genotypes. Understanding both biochemical and functional phenotypes of outlier mutations will boost our insights into CFTR folding and misfolding, and lead to improved therapeutic strategies.
Tamara Hillenaar is in the Dept of Cellular Protein Chemistry, Bijvoet Centre for Biomolecular Research, Science for Life, Faculty of Science, Utrecht University, 3584 CS Utrecht, The Netherlands.
Raksha Jain, Alvit Wolf, Michal Molad, Jennifer Taylor-Cousar, Charles R Esther Jr , Michal Shteinberg. Congenital bilateral cataracts in newborns exposed to elexacaftor-tezacaftor-ivacaftor in utero and while breast feeding. J Cyst Fibros. 2022 Nov;21(6):1074-1076. doi: 10.1016/j.jcf.2022.10.004. Epub 2022 Oct 18. pubmed.ncbi.nlm.nih.gov/36266182/
Elexacaftor-tezacaftor-ivacaftor (ETI) is known to pass through the placenta and into breast milk in mothers who continue on this therapy while pregnant and breast feeding. Toxicity studies of ivacaftor in rats demonstrated infant cataracts, but cataracts were not reported in human infants exposed to ivacaftor. We describe 3 cases of infants exposed to elexacaftor-tezacaftor-ivacaftor (ETI) in utero and while breast feeding who were found to have bilateral congenital cataracts within six months of birth. None of the infants had significant visual impairment from the cataracts nor any report of elevated liver function testing. These data highlight the need to counsel females who continue ETI throughout pregnancy and while breast feeding to consider cataract screen for their infants.University of Texas Southwestern Medical Center, Dallas, TX, USA.
Brionna N Hudson, Hollyann R Jacobs, Alexander Philbrick, Xiaolong A Zhou, Michelle M Simonsen, Julie A Safirstein, Shannon M Rotolo.Drug-induced acne with elexacaftor/tezacaftor/ivacaftor in people with cysticfibrosis. J Cyst Fibros 2022 Sep 7;S1569-1993(22)00657-9.doi: 10.1016/j.jcf.2022.09.002.Online ahead of print. pubmed.ncbi.nlm.nih.gov/36088208/

Brianna S Hudson
linkedin,com
Elexacaftor/tezacaftor/ivacaftor (ELX-TEZ-IVA) is a Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulator shown to improve lung function and reduce sweat chloride in people with Cystic Fibrosis (CF). The only commonly reported dermatologic adverse effect with CFTR modulators including ELX-TEZ-IVA is rash. In this case series, we describe 19 patients who reported new onset or worsening of acne after initiation of this drug to their CF pharmacist or another member of their CF care team. The mechanism and frequency of this adverse effect is unknown.
Dr Brionna N Hudson is at the College of Pharmacy Chicago State University
Divyalakshmi Bhaskaran, Kathryn Bateman. A case of Elexacaftor-Tezacaftor-Ivacaftor induced rash resolving without interruption of treatment. Case Reports J Cyst Fibros. 2022 Nov;21(6):1077-1079. doi: 10.1016/j.jcf.2022.06.011. Epub 2022 Jul 12. pubmed.ncbi.nlm.nih.gov/35840534/

Kathryn Bateman
The triple combination of Elexacaftor-Tezacaftor-Ivacaftor (ELX-TEZ-IVA) has been shown to markedly improve lung function in persons with cystic fibrosis (pwCF). An important adverse effect of the drug is rash, which was reported in clinical trials and highlighted in case reports. Our report demonstrates a similar adverse event with the drug in one of our patients with spontaneous resolution of the rash not necessitating cessation of treatment or desensitization to the drug as were done in other cases. We highlight through our report the heterogeneity of the clinical presentation of the rash when on triple therapy and the need for further studies to understand the immunological mechanism of this adverse event.
Divyalakshmi Bhaskaran is NIHR Academic Clinical Fellow, Leeds Teaching Hospitals NHS Foundation Trust, United Kingdom.
Kathryn Bateman is Cystic Fibrosis/Respiratory Medicine Consultant, Bristol Adult Cystic Fibrosis Centre, United Kingdom.
Marcus A Mall , Rossa Brugha , Silvia Gartner , Julian Legg, Alexander Moeller, Pedro Mondejar-Lopez, Dario Prais,, Tacjana Pressle , Felix Ratjen , Philippe Reix , Paul D Robinson, Hiran Selvadurai, Florian Stehling, Neil Ahluwalia , Emilio Arteaga-Solis, Bote G Bruinsma, Mark Jenning , Samuel M Moskowitz , Sabrina Noel , Simon Tian , Tanya G Weinstock , Pan Wu , Claire E Wainwright , Jane C Davies. Efficacy and Safety of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 Through 11 Years of Age with Cystic Fibrosis Heterozygous for F508del and a Minimal Function Mutation: A Phase 3b, Randomized, Placebo-controlled Study. Am J Respir Crit Care Med. 2022 Dec 1;206(11):1361-1369. doi: 10.1164/rccm.202202-0392OC Free PMC article /pubmed.ncbi.nlm.nih.gov/35816621/

Marcus A Mall
Rationale: The triple-combination regimen elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was shown to be safe and efficacious in children aged 6 through 11 years with cystic fibrosis and at least one F508del-CFTR allele in a phase 3, open-label, single-arm study.
Objectives: To further evaluate the efficacy and safety of ELX/TEZ/IVA in children 6 through 11 years of age with cystic fibrosis heterozygous for F508del and a minimal function CFTR mutation (F/MF genotypes) in a randomized, double-blind, placebo-controlled phase 3b trial. Methods: Children were randomized to receive either ELX/TEZ/IVA (n = 60) or placebo (n = 61) during a 24-week treatment period. The dose of ELX/TEZ/IVA administered was based on weight at screening, with children <30 kg receiving ELX 100 mg once daily, TEZ 50 mg once daily, and IVA 75 mg every 12 hours, and children ⩾30 kg receiving ELX 200 mg once daily, TEZ 100 mg once daily, and IVA 150 mg every 12 hours (adult dose).
Measurements and Main Results: The primary endpoint was absolute change in lung clearance index2.5 from baseline through Week 24. Children given ELX/TEZ/IVA had a mean decrease in lung clearance index2.5 of 2.29 units (95% confidence interval [CI], 1.97-2.60) compared with 0.02 units (95% CI, -0.29 to 0.34) in children given placebo (between-group treatment difference, -2.26 units; 95% CI, -2.71 to -1.81; P < 0.0001). ELX/TEZ/IVA treatment also led to improvements in the secondary endpoint of sweat chloride concentration (between-group treatment difference, -51.2 mmol/L; 95% CI, -55.3 to -47.1) and in the other endpoints of percent predicted FEV1 (between-group treatment difference, 11.0 percentage points; 95% CI, 6.9-15.1) and Cystic Fibrosis Questionnaire-Revised Respiratory domain score (between-group treatment difference, 5.5 points; 95% CI, 1.0-10.0) compared with placebo from baseline through Week 24. The most common adverse events in children receiving ELX/TEZ/IVA were headache and cough (30.0% and 23.3%, respectively); most adverse events were mild or moderate in severity.
Conclusions: In this first randomized, controlled study of a cystic fibrosis transmembrane conductance regulator modulator conducted in children 6 through 11 years of age with F/MF genotypes, ELX/TEZ/IVA treatment led to significant improvements in lung function, as well as robust improvements in respiratory symptoms and cystic fibrosis transmembrane conductance regulator function. ELX/TEZ/IVA was generally safe and well tolerated in this pediatric population with no new safety findings.
Prof. Dr Marcus M Mall is head of the Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin and Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Berlin, Germany. German Center for Lung Research, Associated Partner, Berlin, Germany.
G Spoletini, L Gillgrass, K Pollard, N Shaw, E Williams, C Etherington, I J Clifton, D G Peckham. Dose adjustments of Elexacaftor/Tezacaftor/Ivacaftor in response to mental health side effects in adults with cystic fibrosis. J Cyst Fibros. 2022 Nov;21(6):1061-1065. doi: 10.1016/j.jcf.2022.05.001. Epub 2022 May 16 35585012 Free article pubmed.ncbi.nlm.nih.gov/35585012/

Giulia Spoletini
Introduction: Deterioration in mental health has been reported in a minority of individuals with cystic fibrosis starting elexacafor/tezacaftor/ivacaftor (ELX/TEZ/IVA). We report our experience of using sweat chloride and markers of clinical stability to titrate dose reduction with the aim of minimising adverse events and maintaining clinical stability.
Method: Adults (n = 266) prescribed ELX/TEZ/IVA, were included. Adverse events, sweat chloride, lung function and clinical data were collected.
Results: Nineteen (7.1%) individuals reported anxiety, low mood, insomnia and “brain fog” with reduced attention and concentration span. Thirteen underwent dose reduction with sweat chloride remained normal (<30 mmol l-1) or borderline (30-60 mmol l-1) in six (46.2%) and seven (53.2%) cases respectively. Improvement or resolution of AEs occurring in 10 of the 13 cases.
Conclusion: Dose adjustment of ELX/TEZ/IVA was associated with improvement in mental health AEs without significant clinical deterioration. Sweat chloride concentration may prove useful as a surrogate marker of CFTR function.
Dr G Spoletini is at the Regional Adult CF Centre, St James’s University Hospital, Leeds Teaching Hospital NHS Trust, Leeds, United Kingdom; Leeds Institute of Medical Research, University of Leeds, Leeds, United Kingdom.
Léa Okroglic, Pierre Sohier, Clémence Martin, Coralie Lheure , Nathalie Franck, Isabelle Honoré, Reem Kanaan, Pierre-Régis Burgel , Agnès Carlotti , Nicolas Dupin , Bénédicte Oulès. Acneiform Eruption Following Elexacaftor-Tezacaftor-Ivacaftor Treatment in Patients With Cystic Fibrosis. JAMA Dermatol. 2022 Nov 30;e225208. doi: 10.1001/jamadermatol.2022.5208. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36449298/

Benedicte Oules
Importance: A new treatment for cystic fibrosis combining 3 CFTR modulators- (ELX), tezacaftor (TEZ), and ivacaftor (IVA)-has recently been approved for cystic fibrosis treatment. The cutaneous adverse effects following treatment with this combination are poorly described in the literature.
Objective: To describe the clinicopathological features and treatment response of ELX-TEZ-IVA-associated acneiform eruptions in patients with cystic fibrosis.
Design, setting, and participants: This case series study was conducted in the Dermatology Department of Cochin Hospital, Paris, France, from July 2021 to June 2022 in collaboration with the Cochin Reference Center for Cystic Fibrosis. Referred patients were examined by senior dermatologists. All patients with cystic fibrosis treated with ELX-TEZ-IVA and referred for an acneiform rash were included.
Exposures: Treatment with ELX-TEZ-IVA.
Main outcomes and measures: Onset of acneiform rash, type of lesions, and degree of severity, as well as treatments initiated and response, were evaluated. When performed, skin biopsies were reviewed.
Results: This study included 16 patients (11 women [68.7%]) with a median (range) age of 27 (22-38) years. Six patients (37.5%) developed new-onset acneiform rashes, whereas 10 patients (62.5%) had a relapse (5 patients) or worsening (5 patients) of previous acne. The median (range) onset of acneiform rash was 45 (15-150) days. At inclusion, 11 patients (68.7%) had facial hyperseborrhea, 15 patients (93.7%) had noninflammatory lesions, and 14 (87.5%) had inflammatory lesions of seborrheic regions. Four patients (25.0%) had severe acne with deep inflammatory lesions and pitted scars. A specific pathological pattern of necrotizing infundibular crystalline folliculitis was observed in 4 patients. Topical acne treatments, antibiotics, and isotretinoin were used successfully in these patients, resulting in partial or complete remission in 12 patients (85.7% of patients reevaluated).
Conclusions and relevance: This case series study found that acneiform eruption is an adverse event associated with ELX-TEZ-IVA treatment in patients with cystic fibrosis. Most patients developed mild lesions. However, isotretinoin treatment may be necessary in some patients. The mechanism of ELX-TEZ-IVA-associated acneiform eruption is currently unknown, but the observation of necrotizing infundibular crystalline folliculitis in biopsied patients may guide further exploration.
Léa Okroglic is in the Dept. of Dermatology, Hôpital Cochin, AP-HP, AP-HP.Centre-Université Paris Cité, Paris, France.
Daniel H Tewkesbury, Varinder Athwal, Rowland J Bright-Thomas, Andrew M Jones, Peter J Barry Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on liver tests at a large single adult cystic fibrosis centre. J Cyst Fibros. 2023 Jan 18;S1569-1993(23)00008-5. doi: 10.1016/j.jcf.2023.01.007. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36669962/
Background: Elexacaftor/tezacaftor/ivacaftor (E/T/I) therapy has resulted in substantial improvements in health status for many with cystic fibrosis. Monitoring of liver tests is recommended due to observed rises in transaminases in trials and cases of hepatotoxicity. Comprehensive data in large populations of unselected individuals and those with established CF related liver disease (CFLD) is lacking.
Methods: Patients prescribed E/T/I at a large, adult centre had liver tests monitored at least 3 monthly for 12 months. Changes in individual liver tests were analysed and abnormalities were compared in those with and without CFLD.
Results: 255 of 267 eligible patients were included. Mild rises in median ALT, AST and bilirubin from baseline to 3 months (all p < 0.001) within normal limits were noted which were sustained. There were no differences in changes in liver tests between those with or without CFLD. There was a significant difference in alkaline phosphatase for those with raised levels at baseline versus those with normal baseline level (-18.5 vs +2.0 IU/L, p = 0.002). Clinically significant rises in ALT and AST occurred in 8 (3.1%) and 6 (2.4%) cases respectively, with derangements in 2 individuals attributed to therapy.
Conclusions: E/T/I leads to a mild, likely clinically insignificant increase in ALT, AST and bilirubin after 3 months which is sustained but does not appear to increase further in the vast majority. Underlying CFLD should not be a barrier to treatment. Although there was a reduction in ALP when elevated at baseline, this was not unique to those with pre-existing CFLD.
Daniel H Tewkesbury is at the Manchester University NHS Foundation Trust, Manchester, UK; Division of Immunology, Immunity to Infection & Respiratory Medicine, University of Manchester, Manchester, UK.
Steven Levitte, Yonathan Fuchs, Russell Wise , Zachary M Sellers. Effects of CFTR modulators on serum biomarkers of liver fibrosis in children with cystic fibrosis. J Hepatol Commun. 2023 Jan 20;7(2):e0010. doi: 10.1097/HC9.0000000000000010. Free article pubmed.ncbi.nlm.nih.gov/36662672/

Steven Levitte. LinkedIn
The cystic fibrosis (CF) transmembrane conductance regulator corrector/potentiator combinations lumacaftor/ivacaftor and elexacaftor/tezacaftor/ivacaftor improve sweat chloride, pulmonary function, and nutrition. Yet it is unclear whether they may also impact the progression of liver fibrosis, which is a substantial source of morbidity and mortality for patients with CF. We conducted a retrospective, single-center analysis of children and adolescents with CF treated with lumacaftor/ivacaftor and/or elexacaftor/tezacaftor/ivacaftor therapy, focusing on alterations in liver function tests and fibrosis indices using previously-established thresholds that corresponded with increased liver elastography.
In pairwise comparisons of before and during treatment timepoints, we found that those with CF-associated liver involvement experienced significant decreases in gamma-glutamyl transferase, aspartate aminotransferase-to-platelet index, and gamma-glutamyl transferase-to-platelet ratio while on lumacaftor/ivacaftor. These differences were not observed in patients treated with elexacaftor/tezacaftor/ivacaftor, nor were they observed in patients without underlying CF-associated liver disease. These results provide the first evidence that lumacaftor/ivacaftor may improve liver fibrosis in children and adolescents with CF and suggest it may be beneficial in the treatment of CF-associated liver disease
Steven Levitt is in the Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Stanford Univ
Debanjali Purkayastha, Kyla Agtarap, Kristy Wong, Onella Pereira, Jannie Co, Smita Pakhale, Salmaan Kanji. Drug-drug interactions with CFTR modulator therapy in cystic fibrosis: Focus on Trikafta®/Kaftrio®. J Cyst Fibros. 2023 Jan 16;S1569-1993(23)00004-8. doi: 10.1016/j.jcf.2023.01.005. Online ahead of print.pubmed.ncbi.nlm.nih.gov/36653239/

Debanjali Purkayastha LinkedIn
The combination of CFTR modulators ivacaftor, tezacaftor and elexacaftor (Trikafta®, Kaftrio®) significantly improve outcomes, including survival in a broad range of cystic fibrosis patients. These drugs have complicated metabolic profiles that make the potential for drug interactions an important consideration for prescribers, care providers and patients. Prolonged survival also increases risk of age-related disease and their associated pharmacotherapy, further increasing the risk of drug interactions and the need for increased vigilance amongst care providers. We systematically searched the literature for studies identifying and evaluating pharmacokinetic and pharmacodynamic drug interactions involving the components of Trikafta®/Kaftrio®. We also searched electronic databases of drugs for possible drug interactions based on metabolic profiles. We identified 86 potential drug interactions of which 13 were supported by 14 studies. There is a significant need for research to describe the likelihood, magnitude and clinical impact of the drug interactions proposed here.
Debanjali Purkayastha is a Pharmacy Resident at the University of Waterloo, Kitchener, ON, Canada.
Karen S McCoy , Jill Blind , Terri Johnson, Patti Olson, Laura Raterman , Shasha Bai, Mariah Eisner, Shahid I Sheikh, Stephan Druhan, Cody Young, Kimberly Pasley. Clinical change 2 years from start of elexacaftor-tezacaftor-ivacaftor in severe cystic fibrosis .Pediatr Pulmonol 2023 Jan 17. doi: 10.1002/ppul.26318. Online ahead of print. hpubmed.ncbi.nlm.nih.gov/36650567/

Karen S McCoy. ResearchGate
Rationale: Limited published research is available on the impact of elexacaftor/tezacaftor/ivacaftor (ETI) beyond the initial few months postdrug initiation, especially for those who initiated therapy via individual investigational new drug application. The experiences of patients with cystic fibrosis (CF) experiencing severe lung disease were reviewed for significant improvements in clinical symptoms and quality of life.
Objectives: To examine clinical outcomes 2 years post-ETI in patients with CF and advanced lung disease.
Methods: This single center institutional review board-approved, retrospective chart review assessed clinical markers (percent predicted forced expiratory volume in 1 s, weight, sweat chloride), quality of life and computed tomography scans in patients with advanced lung disease who met criteria for compassionate use/expanded access program due to high risk of death or transplant need within 2 years.
Results: Eighteen identified patients (ages 15-49 years) initiated drug between July and September 2019. Clinical markers indicated that therapy was well tolerated, not discontinued by any participant, and lab values did not indicate medical concern or discontinuation. Monitoring results indicated the safety of modulator therapy as there were no adverse clinical occurrences and all patients presented universal stabilization. There were no deaths and no transplants by the end of the study.
Conclusions: This study focused on patients with CF eligible for modulator therapy and were initiated due to advanced lung disease. Initiation of modulator therapy was deemed safe and resulted in objective positive changes in nutrition, cough, FEV1 , subjective reports of clinical status, level of activity, and a reduction in burden of treatment.
Karen S McCoy is professor at the Pulmonary and Sleep Medicine Division, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Carmen Streibel, Corin C Willers , Orso Pusterla , Grzegorz Bauman, Enno Stranzinger, Ben Brabandt , Oliver Bieri, Marion Curdy, Marina Bullo, Bettina Sarah Frauchiger , Insa Korten, Linn Krüger, Carmen Casaulta, Felix Ratjen, Philipp Latzin, Elisabeth Kieninger. Effects of elexacaftor/tezacaftor/ivacaftor therapy in children with cystic fibrosis – a comprehensive assessment using lung clearance index, spirometry, and functional and structural lung MRI . J Cyst Fibros. 2023 Jan 10;S1569-1993(22)01432-1. doi: 10.1016/j.jcf.2022.12.012. Online ahead of print. pubmed.ncbi.nlm.nih.gov/36635199/

Carmen Streibel
Background: With improvement in supportive therapies and the introduction of cystic fibrosis transmembrane conductance regulator (CFTR)-modulator treatment in patients with cystic fibrosis (CF), milder disease courses are expected. Therefore, sensitive parameters are needed to monitor disease course and effects of CFTR-modulators. Functional lung MRI using matrix-pencil decomposition (MP-MRI) is a promising tool for assessing ventilation and perfusion quantitatively. This study aimed to assess the treatment effect of elexacaftor/tezacaftor/ivacaftor combination regimen (ELX/TEZ/IVA) on measures of structural and functional lung abnormalities.
Methods: 24 children with CF underwent lung function tests (multiple breath washout, spirometry), functional and structural MRI twice (one year apart) before and once after at least two weeks (mean 4.7 ± 2.6 months) on ELX/TEZ/IVA. Main outcomes were changes (Δ) upon ELX/TEZ/IVA in lung function, defect percentage of ventilation (VDP) and perfusion (QDP), defect distribution index of ventilation and perfusion (DDIV, DDIQ), and Eichinger score. Statistical analyses were performed using paired t-tests and multilevel regression models with bootstrapping.
Results: We observed a significant improvement in lung function, structural and functional MRI parameters upon ELX/TEZ/IVA treatment (mean; 95%-CI): ΔLCI2.5 (TO) -0.84 (-1.62 to -0.06); ΔFEV1 (z-score) 1.05 (0.56 to 1.55); ΔVDP (% of impairment) -6.00 (-8.44 to -3.55); ΔQDP (% of impairment) -3.90 (-5.90 to -1.90); ΔDDIV -1.38 (-2.22 to -0.53); ΔDDIQ -0.31 (-0.73 to 0.12); ΔEichinger score -3.89 (-5.05 to -2.72).
Conclusions: Besides lung function tests, functional and structural MRI is a suitable tool to monitor treatment response of ELX/TEZ/IVA therapy, and seems promising as outcome marker in the future.
Carmen Streibel is in the Dept of Paediatric Respiratory Medicine and Allergology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Switzerland.Division of Paediatric Respiratory Medicine and Allergology, Department of Paediatrics, Inselspital, Bern University Hospital, University of Bern, Switzerland.

